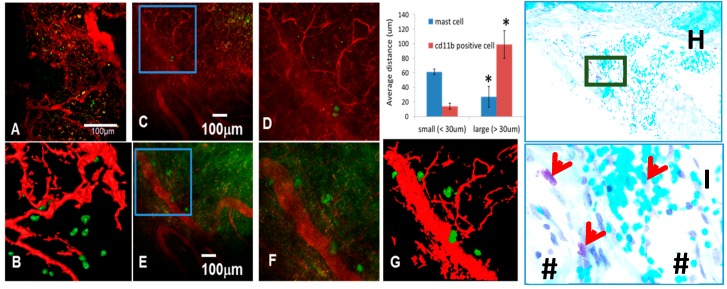
Figure 1.
Mast cells accumulate proximal to large vessels in fibrotic tissue of healing critical defects. The murine chronic cranial defect window model was utilized for in vivo multiphoton laser scanning microscopy (MPLSM) to assess the temporal-spatial relationship of arteriogenesis and mast cell accumulation during critical bone defect healing. MPLSM was performed on mice (n = 5) with cranial defect windows following administration of i.v. Texas red dextran (TRD) and APC-conjugated anti-CD11b or FITC conjugated anti-Mcpt5 antibodies. (A) Representative 10× field of the CD11b+ cells (green) and vasculature (red) with a 3D reconstruction (B), are shown to illustrate that monocytes and macrophages primarily exist proximal to small vessels within the critical defect after three weeks of healing; In contrast, few Mcpt5+ mast cells are found near small vessels ((C); boxed region is (D)); but appear in immediate proximity to large vessels ((E); boxed region is (F) and 3D reconstructed image is (G)); MATLAB quantification of the distance of the labeled cells from small and large vessels is presented as mean +/− SD (* p < 0.05 vs. small vessels). Histologic confirmation of these findings is provided by toluidine blue staining of the calvaria tissue presented at 5× (H); and boxed region at 20× (I); in which the granulated mast cells (red arrows) stain purple in immediate proximity to large blood vessels (#).