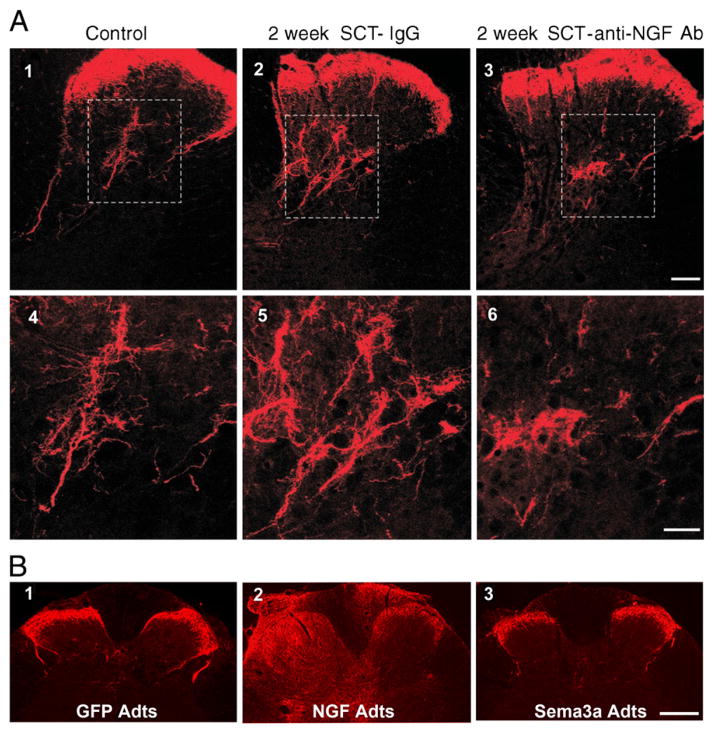
Fig. 1.
Photomicrographs of CGRP-immunoreactive fibers in the dorsal horn of a control intact rat (A1) and of rats 2 weeks after cord transection at the 4th thoracic (T4) segment (A2-6 and B1-3). A) Cord-injured rats received an intrathecal infusion of nonimmune rabbit IgG or an intrathecal infusion of anti-NGF Ab. Transverse tissue sections are from the T6 segment. Panels 4–6 are magnifications of laminae III–V from panels 1–3 (boxed area). Scale bars: 1–3, 100 μm; 3–6, 50 μm. The anti-NGF treatment markedly decreased the size of the CGRP+ arbor in the deeper laminae. [Adapted, with permission, from (Krenz et al., 1999)]. B) Immediately after cord injury adenoviral vectors encoding green fluorescent protein (GFP adts, B1), or NGF (NGF Adts, B2) or Semaphorin 3A (sema3A adts, B3) were injected into the lumbosacral spinal cord. These sections of the L6/S1 cord show that NGF overexpression induced CGRP+ fiber sprouting throughout the dorsal horns and even dorsal columns. Conversely, Sema3A reduced post-traumatic aberrant CGRP fiber sprouting into the dorsal horn. Scale bar: 200 μm. [Adapted, with permission, from (Cameron et al., 2006)].