Abstract
Objective
To determine the impact of endometriotic lesion removal on local and systemic inflammation.
Design
Multiplex cytokine analysis on samples from endometriosis patients before surgery, 2 weeks after surgery, and 3 months after surgery.
Setting
Academic teaching hospital and university.
Patient(s)
A total of 43 endometriosis patients before and after excision of lesions by means of laparoscopic surgery, and 25 normal women.
Intervention(s)
None.
Main Outcome Measure(s)
Plasma, eutopic and ectopic tissue, and peritoneal fluid cytokine levels.
Result(s)
Compared with presurgery plasma samples, levels of granulocyte-macrophage colony-stimulating factor (GM-CSF), interleukin (IL) 2, IL-8, and IL-10 decreased significantly by 2 weeks after surgery in endometriosis patients. Interestingly, levels began to rise at 3 months after surgery in most cases. In tissue, levels of GM-CSF and IL-15 were lower in eutopic tissue, while levels of basic fibroblast growth factor, interferon-inducible protein 10, IL-1 receptor antagonist, granulocyte colony–stimulating factor, macrophage inflammatory protein 1β, IL-7, and IL-5 were higher in eutopic than in ectopic tissue. In peritoneal fluid, levels of IL-5 and IL-12 were higher in early versus advanced stages of endometriosis. Compared with normal women, plasma from endometriosis patients had higher levels of inflammatory cytokines.
Conclusion(s)
Endometriotic lesion removal significantly alters the inflammatory profile both locally and systemically in women with endometriosis. Our findings indicate that ectopic lesions are the major drivers of systemic inflammation in endometriosis. The transitory nature of the change may reflect the recurrence of the condition and the influence of systemic factors in its onset.
Keywords: Cytokines, inflammation, plasma, endometriosis
Endometriosis is a gynecologic condition characterized by the presence and growth of endometrial tissue outside of the uterus, predominantly in the peritoneal cavity. The condition is thought to affect ~5%–10% of women of reproductive age, but is present in up to 70% of women with infertility or pelvic pain (1, 2). Although the symptoms are debilitating in many patients, endometriosis can be asymptomatic, and symptoms do not always correlate to the severity of the disease. The associated burden on the affected patients arises mainly due to pain and subfertility for which the limited treatment options often target the symptoms rather than the underlying condition (3). The cause and mechanism of progress of endometriosis are yet unknown. Retrograde menstruation is the most accepted theory on the pathogenesis of endometriosis, although the predominance of the phenomenon suggests that additional susceptibility factors must be present for the development of the condition.
The growth of endometriotic lesions seems to depend on an inflammatory environment and an aberrant immune response, both of which can contribute to the growth of ectopic tissue via the stimulation of other aggravating processes (2). Previous studies have identified the overexpression of proinflammatory cytokines and growth factors in the peritoneal fluid (PF) and tissues of endometriosis patients, while simultaneously finding decreased levels of antiinflammatory cytokines (4). Endometriosis is also characterized by estrogen dependence and progesterone resistance (5, 6), meaning that lesions manage to evade the cyclic controls that regulate normal endometrial function. The local production of these hormones has been observed in endometriosis patients and seems to be promoted by aberrant cytokine expression (7, 8). Additionally, the sites of ectopic endometrial growth have been shown to undergo significant immune infiltration (9–11), likely due to the higher levels of chemokines expressed in the peritoneal cavity of these patients (12). Compared with normal women, those with endometriosis not only have altered numbers of infiltrating immune cells, but these cells often exhibit reduced cytotoxicity (13–15), meaning that endometriosis patients have an impaired capacity to clear the ectopic endometrial fragments. This aberrant immune environment not only exacerbates the inflammation, but also helps to promote the growth and vascularization of lesions via dysregulated expression of growth factors and angiogenic cytokines (16–18).
Because cytokines are the signaling molecules of the immune system, aberrant cytokine signaling is both an indicator and a regulatory agent of these processes. The complexity of the molecular pathways that regulate inflammation and the immune response presents a great challenge to researchers, with most studies highlighting the role of one or several key regulatory factors, and none providing a definitive basis to the pathogenesis of endometriosis. Previous studies also fail to elucidate whether local inflammation is a trigger or a consequence of endometrial lesion establishment and growth. In the present study, we sought to determine the contribution of the lesions to the local and systemic inflammatory profile in women with endometriosis. We examined the profile of 26 cytokines in the plasma, eutopic and ectopic tissue, and PF of women with endometriosis. We followed endometriosis patients who underwent surgery to remove the lesions and measured the levels of cytokines before surgery, 2 weeks after surgery, and 3 months after surgery. To our knowledge, this is the first report to examine the impact of endometriotic lesion removal on local and systemic cytokine levels. We also compared plasma samples from endometriosis patients with those of normal women, to determine if there were relevant differences in their cytokine profiles.
MATERIALS AND METHODS
Ethics Statement
Ethics approval for this study was obtained from the Greenville Health System (South Carolina) and the University of North Carolina (Chapel Hill), and written informed consent from every subject was obtained before sample collection and storage (IRB protocol no. Pro00000993).
Sample Collection, Patient Characteristics, and Inclusion/Exclusion Criteria
Samples were obtained from 43 patients with visually diagnosed endometriosis undergoing resection of lesions because of infertility or pain at Greenville Hospital. Patients from whom we obtained plasma samples (n = 19) were further subgrouped into early (stages I and II; n = 14) and advanced (stages III and IV; n = 5) disease categories. Subjects with endometriosis were recruited and consented at the preoperative visit. Subjects were recruited if they were undergoing diagnostic laparoscopy and included if endometriosis was clinically identified at the time of surgery. All of the endometriosis patients included in this study underwent surgery. Stage of endometriosis was assigned by the surgeon based on the revised American Society for Reproductive Medicine criteria (19). Blood was drawn before surgery for collection of plasma in an EDTA-containing vacuum tube. Endometrial biopsy was performed after uterine lavage and cervical brushings. Laparoscopy was performed as part of the diagnostic evaluation for infertility and/or pelvic pain. Before resection or any surgical ablation or hysteroscopy, PF was obtained with the use of a cannula and syringe. At the 2-week postoperative visit and at 3 months, venous blood sampling was repeated. Women on oral contraceptives or injectable progestins were excluded from the study. Only women of reproductive age without hormone treatment in the preceding 3 months were included. We also obtained plasma samples from 25 apparently healthy normally cycling women volunteers aged 20–36 years from the University of North Carolina School of Medicine. They were recruited by advertising in the local media and screened according to study guidelines. Clinical information was obtained with the use of a standardized questionnaire reviewed by the study coordinator. None of the healthy volunteers had signs or symptoms of endometriosis or infertility-related problems.
Protein Extraction
Eutopic and ectopic samples obtained from endometriosis patients were used for total protein extraction. Briefly, tissue samples were placed in 1.5-mL microcentrifuge tubes containing 200 µL phosphate buffered saline solution. The protease inhibitor aprotonin was added to each tube (2 µL; Sigma Aldrich). The tissue was homogenized with the use of a rotorstator homogenizer on ice. The samples were then centrifuged at 4°C and the supernate collected. The samples were diluted to a protein concentration of 5 µg/µL with the use of DNAse/RNAse–free water and subsequently stored at −80°C. Protein concentrations were determined with the use of a protein assay (Bio-Rad).
Cytokine Multiplex Analysis
A single panel of a commercial multiplex assay (Luminex xMAP laser bead technology) available from Bio-Rad was used for analysis of selected cytokines/chemokines involved in the inflammatory and angiogenic pathways: platelet-derived growth factor (PDGF) BB, Eotaxin, basic fibroblast growth factor (bFGF), granulocyte colony–stimulating factor (G-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF), interferon (IFN) γ, interferon-inducible protein (IP) 10, monocyte chemotactic protein (MCP) 1, macrophage inflammatory protein (MIP) 1α, MIP-1β, Regulated on activation, normal T expressed and secreted (RANTES), tumor necrosis factor (TNF) α, vascular endothelial growth factor (VEGF), interleukin (IL) 1β, IL-1 receptor antagonist (IL-1Ra), IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12, IL-13, and IL-15. Briefly, color-coded polystyrene beads were coupled with capture antibodies for each respective target cytokine. Plasma samples were aliquotted into 15 µL and diluted four times with the use of the provided sample diluent. Diluted plasma and protein samples were preincubated with 50 µL antibodies conjugated to magnetic beads in a 96-well plate. The plate was covered and incubated in the dark at room temperature on a shaker for 30 minutes. The plate was washed three times with the wash buffer provided with the kit. After diluting 25 µL detection antibody in detection antibody diluent, the plate was incubated in the dark at room temperature on a shaker for 30 minutes. The plate was washed three times in wash buffer, and 50 µL streptavidin-phycoerythrin fluorescent conjugate was added to the wells. The plate was incubated for 10 minutes before being washed, and then a Bio-Plex 200 System was used to read it. The standards for this assay were provided by the manufacturer as a lyophilized cocktail of proteins and were run for each individual target analyte. The cocktail was then serially diluted and standard curve dilutions were run within the assay. Increasing fluorescent intensity signals are read as fluorescence intensity values, which are in direct proportion to protein bound to the specific analyte bead population. Observed concentration for each target analyte was calculated against standard curve regression, and values were recorded in units of pg/mL. Cytokines were quantified through the detection of conjugate, which is in direct proportion to the amount of the target analyte.
Statistical Analysis
Statistical analysis was performed with the use of Sigma Stat 3.5. Plasma samples from endometriosis patients (n = 19) were pooled as well as further subgrouped into early (stages I and II; n = 14) and advanced (stages III and IV; n = 5) disease categories. Plasma samples from normal subjects (n = 25) were pooled as well as subgrouped by menstrual cycle phase into secretory phase (n = 9) and proliferative phase (n = 9). For the subgrouping, we excluded seven samples for which we did not know the menstrual cycle phase. Plasma data from endometriosis patients were analyzed with the use of one-way repeated-measures analysis of variance (ANOVA) and Tukey post hoc test, or with the use of Friedman ANOVA on ranks for nonparametric data. Paired t test or Wilcoxon signed rank test was used to analyze paired tissue data. Plasma data comparing endometriosis and normal subjects were analyzed with the use of Kruskal-Wallis one-way ANOVA and Dunn post hoc test. Peritoneal fluid data were analyzed with the use of one-way ANOVA or Friedman ANOVA on ranks. A P value of <.05 was considered to be significant.
RESULTS
Excision of Lesions Causes a Significant but Transient Shift in Systemic Cytokine Levels
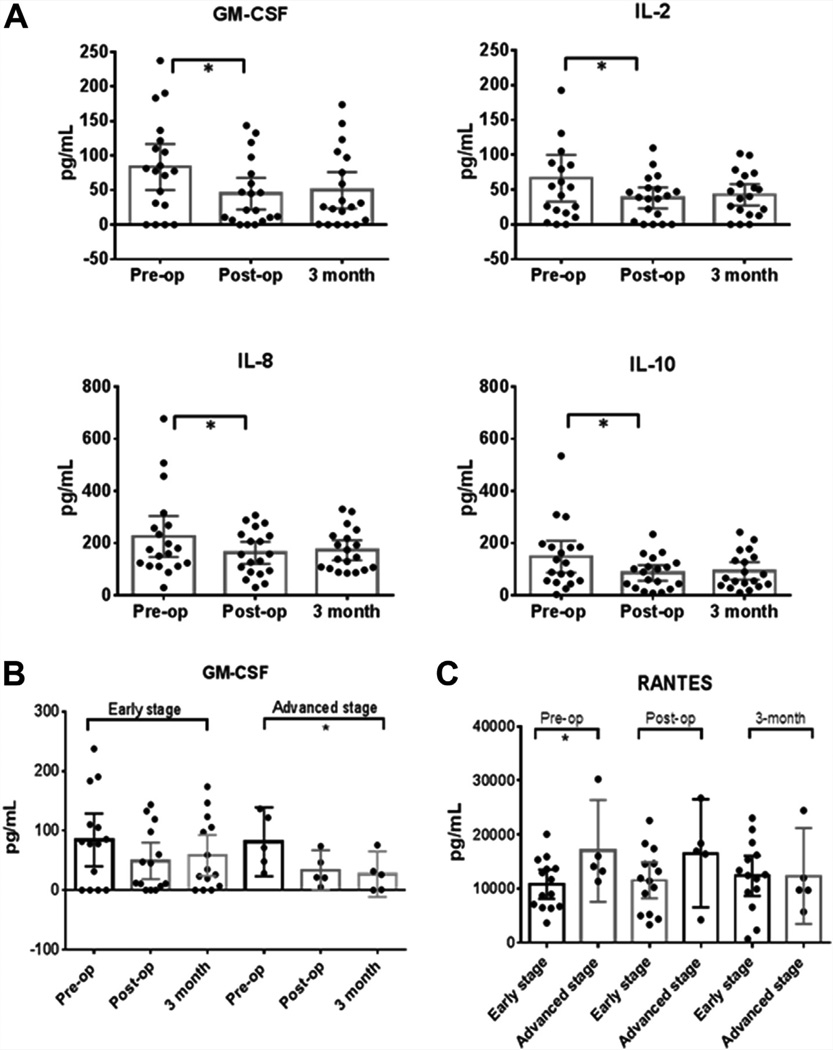
To evaluate the impact of lesion removal on the systemic circulating levels of cytokines, we compared plasma samples obtained from endometriosis patients before surgery, 2 weeks after surgery, and 3 months after surgery. We found that the levels of GM-CSF, IL-2, IL-8, and IL-10 decreased significantly after removal of lesions (Fig. 1A). However, post hoc analysis revealed that this difference was significant only when comparing presurgery versus 2-week postsurgery samples. At 3 months after surgery, the levels of these cytokines were found to increase close to the levels measured before surgery. An overall trend was noted where cytokine levels decreased after surgery in all except four cytokines, although the changes did not achieve statistical significance. The exceptions were IL-12 and IL-13, which remained the same after surgery and were then increased slightly at 3 months after surgery. IP-10 and RANTES seemed to have a delayed decrease: They showed a slight increase 2 weeks after surgery and then a decrease at 3 months after surgery.
FIGURE 1.
(A) Plasma levels of granulocyte-macrophage colony-stimulating factor (GM-CSF), interleukin (IL) 2, IL-8, and IL-10 decreased significantly after removal of lesions. However, post hoc analysis revealed that this difference was significant only in pre- vs. post-op samples, not in pre- vs. 3-month samples. No significant differences were observed for other cytokines. One-way Friedman analysis of variance (ANOVA) and Tukey post hoc test; n = 19. (B) When stratified by disease stage, plasma levels of GM-CSF were found to decrease significantly after surgery in advanced endometriosis patients (stages III–IV), but not in early endometriosis patients (stages I–II). However, post hoc analysis revealed that these differences were not significant. One-way ANOVA and Tukey post hoc test; n = 14 (early stage); n = 5 (advanced stage). (C) When stratified by disease stage and time point, early-stage patients had lower plasma levels of Regulated on activation, normal T expressed and secreted (RANTES) than advanced-stage patients. No differences were seen in early- vs. advanced-stage patients at later time points. No differences were observed for other cytokines. Bars represent the mean, and the whiskers represent the 95% confidence intervals. t test; n = 14 (early stage); n = 5 (advanced stage). *P<.05.
Monsanto. Cytokines in the endometriosis. Fertil Steril 2015.
When the data were stratified by disease stage, GM-CSF levels were found to decrease significantly after surgery in advanced endometriosis patients (stages III–IV), whereas the decrease was not statistically significant in early endometriosis patients (stages I–II). However, post hoc analysis revealed that the differences seen in the advanced stage patients were not significant for any particular pairwise comparison, despite having an overall P value (P=.039) that was significant (Fig. 1B). These results indicate that the decrease in GM-CSF levels was more pronounced in advanced-stage patients than in early-stage patients. No differences were detected for any other cytokine.
When data were stratified by disease stage and time point, RANTES levels were found to be lower in presurgery samples from early-stage patients (stages I–II) compared with presurgery samples from advanced-stage patients (stage III–IV of endometriosis). This means that before surgery, early-stage patients had lower blood levels of RANTES than advanced-stage patients. After surgery, these differences disappeared. No differences were found in 2-week or 3-month postsurgery samples. Also, no differences were observed for any other cytokine (Fig. 1C).
Levels of Inflammatory Cytokines are Predominantly Higher in Endometriosis Patients than in Normally Cycling Women
We performed several comparisons on plasma samples to determine if there were differences between endometriosis patients and normal women and to evaluate the potential influence of such differences in the pathogenesis of the condition. We found 19 different cytokines to be differentially expressed in endometriosis patients compared with normal subjects (Table 1). Interestingly, the levels of 18 of these cytokines were higher in endometriosis patients compared with normal subjects. MCP-1 was the only cytokine expressed at lower levels in endometriosis patients compared with normal subjects, but only when comparing postsurgery samples. When comparing presurgery levels of MCP-1, no significant difference was observed.
TABLE 1.
Comparison of cytokine profiles in plasma samples from endometriosis patients vs. control subjects at each time points.
| Endometriosis (n = 19) | H test | |||||||
|---|---|---|---|---|---|---|---|---|
| Cytokine | Pre-op | Post-op | 3 mo | Normal (n = 25) | df | χ2 | P value | Significancea |
| Eotaxin | 187.23 | 154.12 | 119.30 | 65.11 | 3 | 9.863 | .02 | Post-op > Normal |
| G-CSF | 376.75 | 339.13 | 319.49 | 31.52 | 3 | 31.965 | <.001 | All > Normal |
| IFN-γ | 726.18 | 495.65 | 606.03 | 12.64 | 3 | 23.833 | <.001 | All > Normal |
| IP-10 | 508.33 | 587.68 | 533.15 | 297.42 | 3 | 12.259 | .007 | Post-op > Normal |
| RANTES | 12,937.5 | 13,219.2 | 11,977.6 | 505.96 | 3 | 17.534 | <.001 | All > Normal |
| TNF-α | 244.14 | 193.48 | 209.73 | 10.04 | 3 | 22.551 | <.001 | All > Normal |
| IL-1β | 12.24 | 9.79 | 11.75 | 5.55 | 3 | 8.241 | .041 | Pre-op > Normal |
| IL-1Ra | 862.77 | 565.78 | 659.16 | 51.38 | 3 | 26.179 | <.001 | All > Normal |
| IL-2 | 53.66 | 38.98 | 40.06 | 4.87 | 3 | 10.659 | .014 | Pre-op > Normal |
| IL-4 | 17.21 | 15.99 | 16.18 | 3.90 | 3 | 16.949 | <.001 | All > Normal |
| IL-5 | 33.86 | 25.44 | 23.84 | 0.37 | 3 | 28.976 | <.001 | All > Normal |
| IL-6 | 50.85 | 34.07 | 39.54 | 2.05 | 3 | 21.417 | <.001 | Pre-op > Normal |
| IL-7 | 47.46 | 46.20 | 44.10 | 0.00 | 3 | 24.457 | <.001 | All > Normal |
| IL-8 | 175.70 | 157.22 | 146.65 | 19.29 | 3 | 15.04 | .002 | All > Normal |
| IL-9 | 46.19 | 55.36 | 42.63 | 0.68 | 3 | 24.465 | <.001 | All > Normal |
| IL-10 | 124.87 | 90.10 | 66.18 | 3.99 | 3 | 28.3 | <.001 | All > Normal |
| IL-12 | 98.58 | 125.29 | 88.62 | 6.40 | 3 | 21.265 | <.001 | All > Normal |
| IL-13 | 19.76 | 19.29 | 17.29 | 4.01 | 3 | 12.145 | .007 | Pre-op and 3 mo > Normal |
| MCP-1 | 167.82 | 108.27 | 120.47 | 189.48 | 3 | 10.873 | .012 | Post-op and 3 mo < Normal |
Note: Protein levels are expressed in units of pg/mL.
bFGF = basic fibroblast growth factor; G-CSF = granulocyte colony–stimulating factor; IFN = interferon; IL = interleukin; IL-1Ra = interleukin -1 receptor antagonist; IP = interferon-inducible protein; MCP = monocyte chemotactic protein; RANTES = Regulated on activation, normal T expressed and secreted; TNF = tumor necrosis factor.
Kruskal-Wallis one-way analysis of variance (H test) and Dunn post hoc test.
Monsanto. Cytokines in the endometriosis. Fertil Steril 2015.
When endometriosis data were stratified by disease stage, we found that the levels of 11 cytokines were higher than in normal women (Supplemental Table 1, available online at www.fertstert.org). For all 11 cytokines, the levels were higher only in advanced-stage presurgery samples compared with normal samples, meaning that before surgery, advanced-stage patients had significantly higher levels of these cytokines than normal women, and after surgery the differences disappeared. These differences were not observed in early-stage patients. However, within these 11 cytokines, G-CSF and IL-7 were found to be more highly expressed in early-stage patients at all time points compared with normal women. This means that endometriosis patients had higher levels of G-CSF and IL-7 than normal women regardless of the surgery.
Finally, we compared endometriosis samples with normal samples stratified by phase of menstrual cycle (secretory or proliferative phase), to determine if menstrual cycle fluctuations would influence the comparison (Supplemental Table 2, available online at www.fertstert.org). The results mostly agree with the findings presented in Table 1. We found that 12 out of 18 cytokines were more highly expressed in endometriosis patients at all time points compared with all normal patients. The remaining six cytokines had varying results. Levels of IL-1β and IL-13 were higher in presurgery and 3-month postsurgery samples compared with normal samples from women in the proliferative phase, but not the secretory phase. Eotaxin levels were higher in 2-week postsurgery samples compared with normal proliferative-phase samples only. IL-2 levels were higher in presurgery samples compared with normal proliferative samples.
Inflammatory Cytokines are Differentially Expressed and Related to Disease Stage in Eutopic versus Ectopic Endometrium
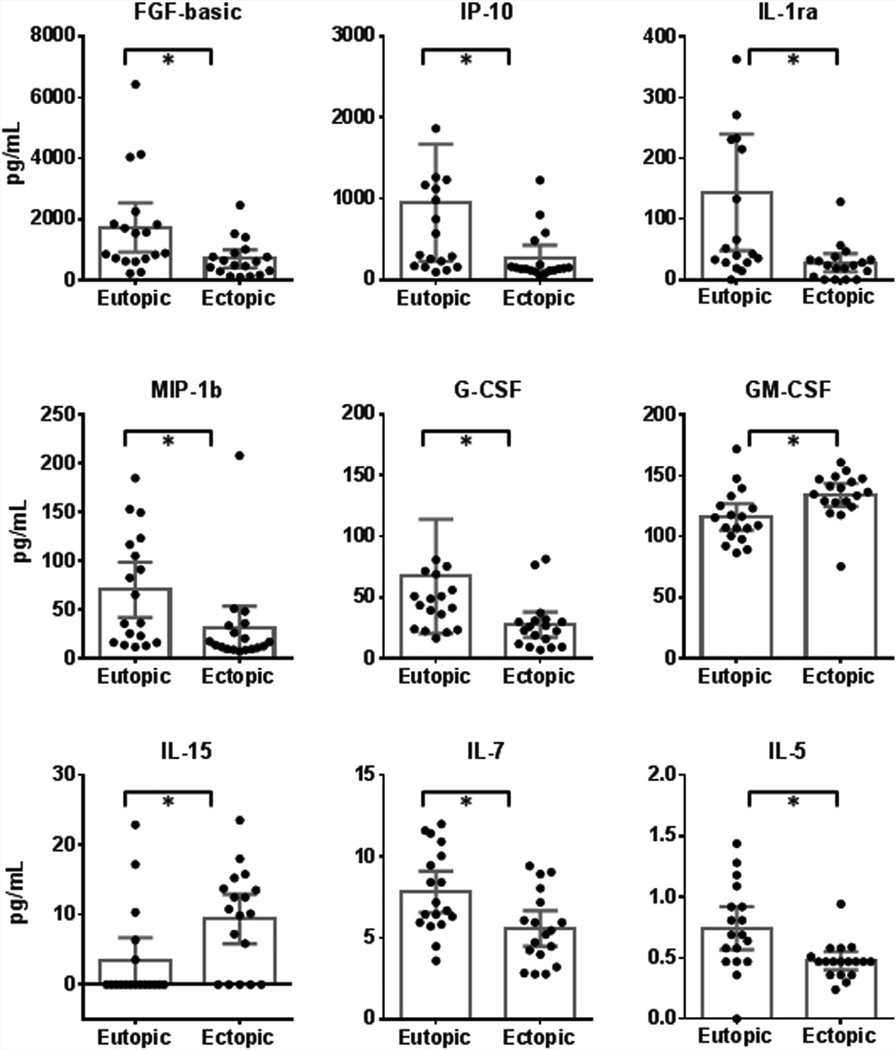
To evaluate the differences between eutopic and ectopic endometrium, we compared cytokine levels between matched eutopic and ectopic tissue obtained from the same patient before surgery (Fig. 2). Differential expression of nine cytokines was detected in eutopic compared with ectopic tissue. Levels of bFGF, IP-10, IL-1Ra, G-CSF, MIP-1β, IL-7, and IL-5 were significantly higher in eutopic than in ectopic tissue. Conversely, levels of GM-CSF and IL-15 were lower in eutopic compared with ectopic tissue.
FIGURE 2.
Differential expression of nine cytokines was detected in eutopic tissue compared with ectopic tissue. Levels of GM-CSF and IL-15 were lower in eutopic tissue. In contrast, levels of basic fibroblast growth factor (FGF-basic), interferon-inducible protein (IP) 10, IL-1 receptor antagonist (IL-1ra), granulocyte colony–stimulating factor (G-CSF), macrophage inflammatory protein (MIP) 1β, IL-7, and IL-5 were higher in eutopic than in ectopic tissue. Other abbreviations as in Figure 1. Bars represent the mean, and the whiskers represent the 95% confidence intervals. *P<.05; paired t test or Wilcoxon signed ranked test for nonparametric data (n = 18).
Monsanto. Cytokines in the endometriosis. Fertil Steril 2015.
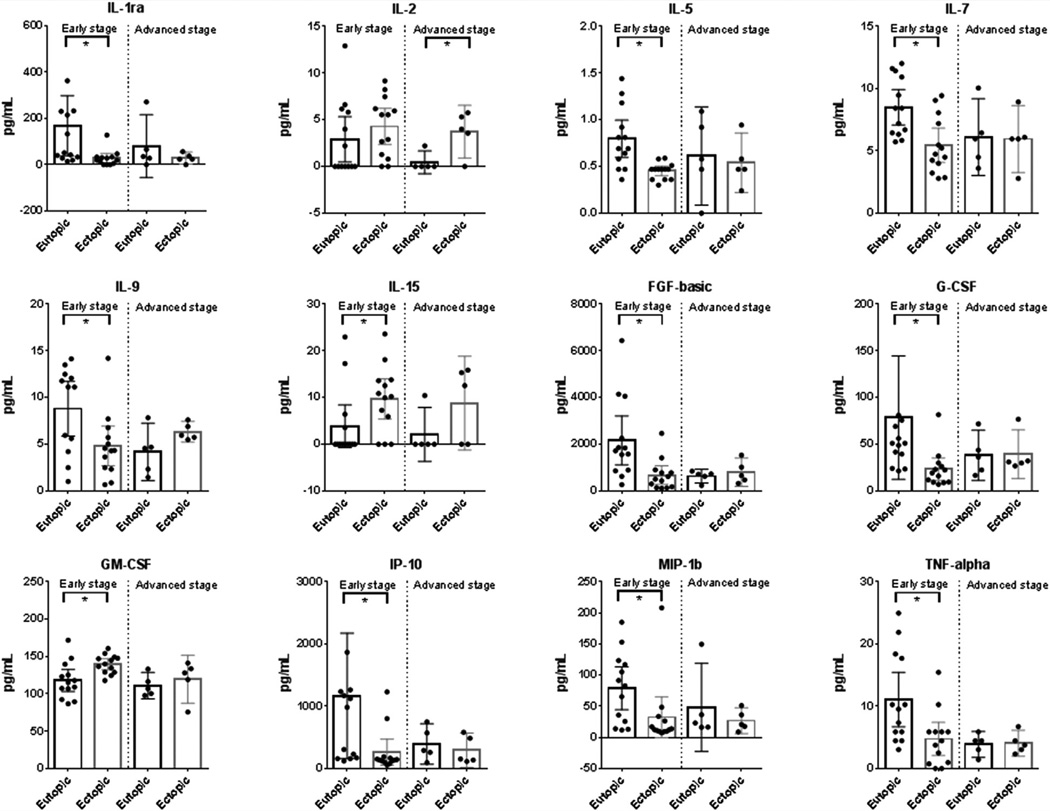
When samples were stratified by disease stage, we detected differential expression of IL-1Ra, IL-5, IL-7, IL-9, IL-15, bFGF, G-CSF, GM-CSF, IP-10, MIP-10, and TNF-α when comparing eutopic versus ectopic tissue in early stage patients. When comparing eutopic and ectopic tissues in advanced-stage patients, only IL-2 was found to be differentially expressed in eutopic versus eutopic tissue (Fig. 3). No differences were observed for the other cytokines.
FIGURE 3.
When samples were stratified by stage of disease, differential expression of IL-1ra, IL-5, IL-7, IL-9, IL-15, FGF-basic, G-CSF, GM-CSF, IP-10, MIP-1β, and tumor necrosis factor (TNF) α was observed between eutopic and ectopic tissues of early-stage patients, but not in advanced-stage patients. Only IL-2 was found to be differentially expressed in eutopic vs. eutopic tissue from advanced-stage patients. Abbreviations as in Figures 1 and 2. Bars represent the mean, and the whiskers represent the 95% confidence intervals. *P<.05; n = 13; paired t test or Wilcoxon signed ranked test.
Monsanto. Cytokines in the endometriosis. Fertil Steril 2015.
When tissue samples were stratified by disease stage and tissue type, levels of PDGF-BB, IL-4, IFN-γ, IL-9, bFGF, and TNF-α were found to be higher in the early-stage eutopic samples than in advanced-stage eutopic samples (Supplemental Fig. 1, available online at www.fertstert.org). When comparing early- versus advanced-stage ectopic samples, GM-CSF levels were higher in early-stage ectopic samples than in advanced-stage ectopic samples. Conversely, ectopic samples had lower G-CSF levels in the early-stage samples than in the advanced-stage samples, but those differences were not seen in the eutopic samples. No differences were observed for the other cytokines.
Peritoneal Fluid Expresses High Levels of Inflammatory Cytokines that Change with Disease Stage
Peritoneal fluid samples from endometriosis patients were compared to evaluate the impact of disease stage on local cytokine levels (Supplemental Fig. 2, available online at www.fertstert.org). IL-5 levels changed significantly when comparing disease stages I, II, and III, but post hoc tests revealed that differences were not significant despite a significant overall P value. IL-12 was also found to change significantly, but only when comparing stage III versus stage I, which could indicate that a significant change occurs in the transition from early to advanced stages. The cytokine profile of PF in these patients was significantly proinflammatory, containing high levels of inflammatory cytokines (Supplemental Fig. 2).
Correlation between Inflammatory Cytokine Levels Across Different Tissues
To determine whether there was an association between cytokine levels in different tissues, we performed a correlation analysis to compare cytokine levels in plasma, tissue, and PF (Supplemental Table 3, available online at www.fertstert.org). We found significant correlations between plasma and ectopic tissue. We found a positive correlation when comparing VEGF levels in presurgery plasma versus ectopic tissue (0.536; P=.0219). When comparing 2-week postsurgery plasma versus ectopic tissue, we found a positive correlation for IL-5 (0.52; P=.0269). When comparing 3-month postsurgery plasma versus ectopic tissue, we found a positive correlation for IP-10 (0.478; P=.0451) and G-CSF (0.499; P=.0352) and a negative correlation for GM-CSF (−0.529; P=.024). Finally, when comparing eutopic versus ectopic tissue, we found a significant negative correlation for IL-4 (−0.658; P=.00301).
DISCUSSION
This study is the first to report that the excision of endometriotic lesions produces a measurable systemic shift in the inflammatory cytokine profile of patients with endometriosis. Inflammation is one of the defining features of endometriosis and has been shown to be a major driving factor for the condition. Our findings provide striking evidence that endometriotic lesions are not simply a result of the condition, but rather act as major regulators and promoters of inflammation, thus aiding the progress of endometriosis at a local and systemic level. Lesion removal resulted in a measurable shift in cytokine levels that extended beyond the local pelvic environment. All except two of the plasma cytokines exhibited a trend of decrease after surgery. Although only four were statistically significant, this trend further supports the idea that endometriotic lesions are the major drivers of inflammation in these patients. Importantly, at 3 months after surgery, cytokine levels seemed to begin to return to their initial levels. The fact that the change was transient suggests that either surgery was unable to remove all traces of the lesions or that additional factors are involved in the onset of endometriosis.
In the pooled plasma analysis, we detected a significant decrease in circulating levels of GM-CSF, IL-2, IL-8, and IL-10 after removal of lesions, which suggests that in these patients, systemic inflammation is driven locally by the ectopic lesions through several mechanisms. IL-10 is mostly known as a key regulatory cytokine essential for the dampening and resolving of inflammation during the immune response. Our findings support previous reports that IL-10 is indeed overexpressed in the serum endometriosis patients (20) and that it is capable of promoting lesion growth by locally suppressing the immune cells that would help clear the endometrial fragments (13, 20). Because IL-10 can inhibit IL-2 and GM-CSF, the increased levels of IL-10 could reflect an attempt to inhibit the high levels of IL-2 and GM-CSF observed in these patients. IL-2 is normally secreted by activated T cells and natural killer cells and is an important regulator of self tolerance (21). The increased expression of IL-2 in plasma may point to the inability of these patients to regulate inflammation and could indicate a predisposition to autoimmune characteristics. GM-CSF is usually secreted in response to other inflammatory cytokines, such as IL-1, and seems to act locally, mediating the recruitment and activation of macrophage-lineage cells (22). We speculate that the high circulating levels of GM-CSF observed in these patients simply reflect an inability to regulate the inflammation at the local level, as supported by the immediate postsurgery decrease in circulating GM-CSF levels. Furthermore, the decrease in GM-CSF levels after surgery was significant in advanced-stage patients and not in early-stage patients. Given that GM-CSF is mostly locally acting, lesions that are larger and more established would be necessary for circulating levels to be influenced. Additionally, ectopic tissue was found to express higher levels of GM-CSF than eutopic tissue, further indicating that ectopic lesions are driving the aberrant expression of this cytokine. Meanwhile, IL-8 is a chemotactic and angiogenic factor previously shown to contribute to neutrophil recruitment and blood vessel formation in endometriotic lesions (23, 24). High levels of IL-8 have previously been detected in the PF and serum of women with endometriosis (25, 26), which supports our findings. However, the fact that IL-8 levels decreased immediately after surgery suggest that the ectopic lesions were the main drivers of these up-regulated levels. IL-8 is also a potent chemotactic factor for basophils and GM-CSF–primed eosinophils (27). Given the high expression of GM-CSF in both plasma and ectopic tissue, IL-8 would aggravate immune infiltration at the lesion sites. Endometrial cells have been shown to produce IL-8 and to have increased expression of IL-8 and cyclooxygenase (COX) 2 under stimulation of IL-17 in vitro (28), meaning they can stimulate local blood vessel formation. Our group recently confirmed that IL-17A is expressed in the eutopic and ectopic endometrium of women with endometriosis, and that IL-17A stimulation of cultured endometrial cells induces the production of VEGF, G-CSF, PDGF-AA, and stromal cell–derived factor 1 (23), which could be a mechanism by which ectopic lesions increase IL-8 production.
To further support the impact of ectopic lesions on systemic inflammation, we detected lower levels of RANTES in the plasma of early-stage patients compared with advanced-stage patients. RANTES is a chemokine involved in the activation and recruitment of immune cells to sites of allergic reaction (29). Given that RANTES is a locally acting chemokine that is also involved in late-phase inflammatory response, we speculate that it may play a more active role in advanced stages of disease, where it would be produced by the larger ectopic lesions and promote immune cell infiltration. Elevated levels of RANTES have been previously detected in the PF and eutopic and ectopic tissues of women with endometriosis (30, 31), and its production can be induced by IL-1β (32). Our analysis of eutopic and eutopic tissue indicates that IL-1 expression is dysregulated at the lesion sites, further supporting previous reports. The combined effect of these factors is a local proangiogenic microenvironment aggravated by unresolved inflammation that leads to a systemic inflammatory response. We also found these results to be supported when we compared plasma from endometriosis patients with that from normal women. Of the 19 cytokines that were statistically significant, 18 were higher in endometriosis patients compared with normal women, including IL-2, IL-8, IL-10, and RANTES. These results indicate that these cytokines are not only dysregulated in endometriosis patients, but also are expressed aberrantly compared with normal subjects. The remaining cytokines have been shown in previous reports to be dysregulated in women with endometriosis, as extensively reviewed elsewhere (33, 34). In our analysis of matched eutopic and ectopic tissue, we found differential expression of nine cytokines, bFGF, IP-10, IL-1Ra, G-CSF, MIP-1β, IL-7, IL-5, GM-CSF, and IL 15, which further supports the idea that this local aberrant cytokine profile is likely driving the systemic inflammation via a positive feedback cycle. Most of these cytokines were more highly expressed in the eutopic than ectopic endometrium. The only exceptions were GM-CSF and IL-15, which were more highly expressed in ectopic tissue. As we previously mentioned, GM-CSF may contribute to the increased inflammatory response, particularly by inducing the differentiation of granulocytes and macrophages or their proliferation at lesion sites.
Conversely, IL-1Ra was more highly expressed in eutopic than in ectopic endometrium. Furthermore, after data stratification, we found that this difference was most significant when comparing matched tissue in early-stage patients, not in advanced-stage patients, which may reflect local dysregulation of IL-1 signaling and an inability to control immunerelated inflammation at an early stage. The IL-1 family, a known key regulator of the inflammatory response, has been found to be aberrantly expressed in women with endometriosis. Active endometriotic lesions seem to have increased expression of the IL-1 type I receptor (35), whereas expression of the decoy IL-1 type II receptor is decreased in the normal endometrium of endometriosis patients compared with normal women (36). These results point to a severely impaired regulation of the proinflammatory signaling of IL-1 in women with endometriosis. We detected higher levels of IL-1β, IL-1Ra, and IL-6 in endometriosis patients than normal women, which supports the notion that there is dysregulation of IL-1 signaling in these patients. For example, IL-1 can induce secretion of IL-6 by fibroblasts, endothelial cells, and circulating monocytes (37) and can stimulate IL-8 secretion by endometriotic cells (24). The decreased expression of IL-1Ra in ectopic tissue would leave IL-1 unregulated and able to promote IL-8 production, as was found in the plasma of patients with endometriosis in this study. IL-6 has been shown to stimulate the secretion of VEGF in isolated peritoneal macrophages and neutrophils from mice (38) and human cell lines (39). Also, high levels of IL-6 have been found in the PF (40) and both eutopic and ectopic endometrium of endometriosis patients compared with normal women (41). The latter study (41) also reported higher levels of IL-1β in the endometrium of these patients, which likely further induces local IL-6 secretion. IL-1β has also been shown to induce neutrophil migration to the peritoneal cavity of rats, and such migration was dependent on macrophages and mast cells (11). Local inflammation is likely sustained by the immune infiltration observed in the pelvic environment of endometriosis patients.
Inflammation can be further aggravated by local production of hormones promoted by aberrant expression of cytokines. For example, TNF-α was one of the cytokines we found to be more highly expressed in endometriosis patients compared with normal subjects. IL-1 and TNF-α are able to induce the expression of COX-2, which mainly produces prostaglandin E2 (PGE2). PGE2 is not only capable of self-induction via up-regulation of COX-2, but can induce aromatase P450 production, which is the key regulatory enzyme in the biosynthesis of estrogens (8). COX-2 has been shown to be overexpressed in the ectopic tissue of patients with endometriosis (42, 43). Furthermore, IL-1Ra, which blocks the IL-1–induced production of COX-2 in endometrial cells (44), is down-regulated in the PF of women with endometriosis (45), which is consistent with our findings that IL-1Ra was down-regulated in ectopic compared with eutopic tissue. PGE2 thus provides a mechanism for lesions to locally sustain the production of prostanoids and estrogens. PGE2 is also capable of altering the phagocytic ability of immune cells and of promoting angiogenesis via VEGF expression (8), further contributing to the survival of lesions in the peritoneal cavity. Meanwhile, IL-5 was one of the cytokines more highly expressed in eutopic versus ectopic tissue, particularly in early-stage patients after data stratification. IL-5 plays a major role in the late-phase allergic response, being produced by mast cells within allergen-challenged tissues (46). Based on our results, we could speculate that IL-5 plays a major role in promoting the local inflammation seen in endometriosis patients, and may play a more important role at earlier stages of disease when lesions are in the process of establishing.
MIP-1β is an inflammatory chemokine that is stimulated by IL-7. Both were more highly expressed in the eutopic endometrium compared with paired ectopic endometrium. IL-7 has been found to be an important immunostimulatory cytokine and to be overexpressed in inflamed tissues of patients with autoimmune diseases such as rheumatic disease (47). IL-7, RANTES, IL-2, and IL-10, all of which were up-regulated in plasma, are cytokines associated with autoimmune conditions and may provide further support for the concept of endometriosis being an autoimmune condition.
Finally, the PF of endometriosis patients in this study expressed high levels of various cytokines, which mostly supports previous reports. The most highly expressed cytokines included Eotaxin, bFGF, G-CSF, GM-CSF, IFN-γ, IP-10, MCP-1, VEGF, IL-1Ra, IL-5, IL-10, and IL-15. Our stage comparisons of IL-5 and IL-12 were marginally significant, so further analyses would be required to understand the true significance of the cytokine profile in the PF of these patients.
CONCLUSION
We provide new insights on the causes of a heightened inflammatory response at local and systemic levels in endometriosis patients. Evidence presented in this manuscript clearly indicates that endometriotic lesions are probably the major drivers of inflammation, because the removal of lesions led to significant reduction in the systemic level of several potent inflammatory factors. Furthermore, most of the cytokines that were dysregulated in plasma or tissue of endometriosis patients were also aberrantly expressed compared with normal subjects. Another important piece of evidence emerging from this study is the striking difference in the immune microenvironment of eutopic and ectopic tissues, because measurable differences were detected when comparing ectopic versus eutopic tissue, as well as the influence of different stages of disease. Although patients recruited in this study were free of any form of therapy for 3 months before surgery to avoid any treatment bias on the cytokine profiles, patients were not tracked specifically for any nonsteroidal antiinflammatory drug (NSAID) use, which should be taken into consideration in future studies. Nevertheless, our results strongly suggest that in endometriosis, an impaired ability to dampen the inflammatory response triggered by the endometrial fragments helps the condition to evolve into a chronic condition. Although our study does not conclusively establish whether inflammation is initiated by the lesions or vice versa, it provides a solid basis that surgical intervention might provide a window of opportunity to dampen inflammation, which would allow for therapeutic interventions, such as reducing subfertility and modulating conditions that permit lesion recurrence.
Supplementary Material
Acknowledgments
Supported by the Canadian Institutes of Health Research (CIHR 394924 to C.T.) and the National Institutes of Health (R01–067721 to S.L.Y. and B.A.L.; P01AT 003961 and P20 GM103641 to P.N. and M.N.).
Footnotes
Discuss: You can discuss this article with its authors and with other ASRM members at http://fertstertforum.com/monsantos-cytokines-endometriosis/
S.P.M. has nothing to disclose. A.K.E. has nothing to disclose. J.Z. has nothing to disclose. P.N. has nothing to disclose. M.N. has nothing to disclose. S.L.Y. has nothing to disclose. B.A.L. has nothing to disclose. C.T. has nothing to disclose.
REFERENCES
- 1.Cramer DW, Missmer SA. The epidemiology of endometriosis. Ann N Y Acad Sci. 2002;955:11–22. doi: 10.1111/j.1749-6632.2002.tb02761.x. discussion 34–6, 396–406. [DOI] [PubMed] [Google Scholar]
- 2.Bulun SE. Endometriosis. N Engl J Med. 2009;360:268–279. doi: 10.1056/NEJMra0804690. [DOI] [PubMed] [Google Scholar]
- 3.Acien P, Velasco I. Endometriosis: a disease that remains enigmatic. ISRN Obstet Gynecol. 2013;2013:242149. doi: 10.1155/2013/242149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gazvani R, Templeton A. Peritoneal environment, cytokines and angiogenesis in the pathophysiology of endometriosis. Reproduction. 2002;123:217–226. doi: 10.1530/rep.0.1230217. [DOI] [PubMed] [Google Scholar]
- 5.Young SL, Lessey BA. Progesterone function in human endometrium: clinical perspectives. Semin Reprod Med. 2010;28:5–16. doi: 10.1055/s-0029-1242988. [DOI] [PubMed] [Google Scholar]
- 6.Lessey BA, Young SL. Homeostasis imbalance in the endometrium of women with implantation defects: the role of estrogen and progesterone. Semin Reprod Med. 2014;32:365–375. doi: 10.1055/s-0034-1376355. [DOI] [PubMed] [Google Scholar]
- 7.Wing LY, Chuang PC, Wu MH, Chen HM, Tsai SJ. Expression and mitogenic effect of fibroblast growth factor-9 in human endometriotic implant is regulated by aberrant production of estrogen. J Clin Endocrinol Metab. 2003;88:5547–5554. doi: 10.1210/jc.2003-030597. [DOI] [PubMed] [Google Scholar]
- 8.Wu MH, Lu CW, Chuang PC, Tsai SJ. Prostaglandin E2: the master of endometriosis? Exp Biol Med. 2010;235:668–677. doi: 10.1258/ebm.2010.009321. [DOI] [PubMed] [Google Scholar]
- 9.Berbic M, Schulke L, Markham R, Tokushige N, Russell P, Fraser IS. Macrophage expression in endometrium of women with and without endometriosis. Hum Reprod. 2009;24:325–332. doi: 10.1093/humrep/den393. [DOI] [PubMed] [Google Scholar]
- 10.Schulke L, Berbic M, Manconi F, Tokushige N, Markham R, Fraser IS. Dendritic cell populations in the eutopic and ectopic endometrium of women with endometriosis. Hum Reprod. 2009;24:1695–1703. doi: 10.1093/humrep/dep071. [DOI] [PubMed] [Google Scholar]
- 11.Oliveira SH, Canetti C, Ribeiro RA, Cunha FQ. Neutrophil migration induced by IL-1beta depends upon LTB4 released by macrophages and upon TNF-alpha and IL-1beta released by mast cells. Inflammation. 2008;31:36–46. doi: 10.1007/s10753-007-9047-x. [DOI] [PubMed] [Google Scholar]
- 12.Borrelli GM, Carvalho KI, Kallas EG, Mechsner S, Baracat EC, Abrao MS. Chemokines in the pathogenesis of endometriosis and infertility. J Reprod Immunol. 2013;98:1–9. doi: 10.1016/j.jri.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 13.Ho HN, Wu MY, Chao KH, Chen CD, Chen SU, Yang YS. Peritoneal interleukin-10 increases with decrease in activated CD4+ T lymphocytes in women with endometriosis. Hum Reprod. 1997;12:2528–2533. doi: 10.1093/humrep/12.11.2528. [DOI] [PubMed] [Google Scholar]
- 14.Kang YJ, Jeung IC, Park A, Park YJ, Jung H, Kim TD, et al. An increased level of IL-6 suppresses NK cell activity in peritoneal fluid of patients with endometriosis via regulation of SHP-2 expression. Hum Reprod. 2014;29:2176–2189. doi: 10.1093/humrep/deu172. [DOI] [PubMed] [Google Scholar]
- 15.Somigliana E, Vigano P, Gaffuri B, Candiani M, Busacca M, Di Blasio AM, et al. Modulation of NK cell lytic function by endometrial secretory factors: potential role in endometriosis. Am J Reprod Immunol. 1996;36:295–300. doi: 10.1111/j.1600-0897.1996.tb00179.x. [DOI] [PubMed] [Google Scholar]
- 16.Bourlev V, Larsson A, Olovsson M. Elevated levels of fibroblast growth factor-2 in serum from women with endometriosis. Am J Obstet Gynecol. 2006;194:755–759. doi: 10.1016/j.ajog.2005.08.064. [DOI] [PubMed] [Google Scholar]
- 17.McLaren J, Prentice A, Charnock-Jones DS, Millican SA, Muller KH, Sharkey AM, et al. Vascular endothelial growth factor is produced by peritoneal fluid macrophages in endometriosis and is regulated by ovarian steroids. J Clin Invest. 1996;98:482–489. doi: 10.1172/JCI118815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Szyllo K, Tchorzewski H, Banasik M, Glowacka E, Lewkowicz P, Kamer-Bartosinska A. The involvement of T lymphocytes in the pathogenesis of endometriotic tissues overgrowth in women with endometriosis. Mediators Inflamm. 2003;12:131–138. doi: 10.1080/0962935031000134842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.American Society for Reproductive Medicine. Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil Steril. 1997;67:817–821. doi: 10.1016/s0015-0282(97)81391-x. [DOI] [PubMed] [Google Scholar]
- 20.Suen JL, Chang Y, Chiu PR, Hsieh TH, Hsi E, Chen YC, et al. Serum level of IL-10 is increased in patients with endometriosis, and IL-10 promotes the growth of lesions in a murine model. Am J Pathol. 2014;184:464–471. doi: 10.1016/j.ajpath.2013.10.023. [DOI] [PubMed] [Google Scholar]
- 21.Nelson BH. IL-2, regulatory T cells, and tolerance. J Immunol. 2004;172:3983–3988. doi: 10.4049/jimmunol.172.7.3983. [DOI] [PubMed] [Google Scholar]
- 22.Hamilton JA. GM-CSF in inflammation and autoimmunity. Trends Immunol. 2002;23:403–408. doi: 10.1016/s1471-4906(02)02260-3. [DOI] [PubMed] [Google Scholar]
- 23.Ahn SH, Edwards AK, Singh SS, Young SL, Lessey BA, Tayade C. IL-17A contributes to the pathogenesis of endometriosis by triggering proinflammatory cytokines and angiogenic growth factors. J Immunol. 2015;195:2591–2600. doi: 10.4049/jimmunol.1501138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akoum A, Lawson C, McColl S, Villeneuve M. Ectopic endometrial cells express high concentrations of interleukin (IL)-8 in vivo regardless of the menstrual cycle phase and respond to oestradiol by up-regulating IL-1–induced IL-8 expression in vitro. Mol Hum Reprod. 2001;7:859–866. doi: 10.1093/molehr/7.9.859. [DOI] [PubMed] [Google Scholar]
- 25.Pizzo A, Salmeri FM, Ardita FV, Sofo V, Tripepi M, Marsico S. Behaviour of cytokine levels in serum and peritoneal fluid of women with endometriosis. Gynecol Obstet Invest. 2002;54:82–87. doi: 10.1159/000067717. [DOI] [PubMed] [Google Scholar]
- 26.Ryan IP, Tseng JF, Schriock ED, Khorram O, Landers DV, Taylor RN. Interleukin-8 concentrations are elevated in peritoneal fluid of women with endometriosis. Fertil Steril. 1995;63:929–932. [PubMed] [Google Scholar]
- 27.Mukaida N, Harada A, Matsushima K. Interleukin-8 (IL-8) and monocyte chemotactic and activating factor (MCAF/MCP-1), chemokines essentially involved in inflammatory and immune reactions. Cytokine Growth Factor Rev. 1998;9:9–23. doi: 10.1016/s1359-6101(97)00022-1. [DOI] [PubMed] [Google Scholar]
- 28.Hirata T, Osuga Y, Hamasaki K, Yoshino O, Ito M, Hasegawa A, et al. Interleukin (IL)-17A stimulates IL-8 secretion, cyclooxygenase-2 expression, and cell proliferation of endometriotic stromal cells. Endocrinology. 2008;149:1260–1267. doi: 10.1210/en.2007-0749. [DOI] [PubMed] [Google Scholar]
- 29.Conti P, DiGioacchino M. MCP-1 and RANTES are mediators of acute and chronic inflammation. Allergy Asthma Proc. 2001;22:133–137. doi: 10.2500/108854101778148737. [DOI] [PubMed] [Google Scholar]
- 30.Khorram O, Taylor RN, Ryan IP, Schall TJ, Landers DV. Peritoneal fluid concentrations of the cytokine RANTES correlate with the severity of endometriosis. Am J Obstet Gynecol. 1993;169:1545–1549. doi: 10.1016/0002-9378(93)90433-j. [DOI] [PubMed] [Google Scholar]
- 31.Wang XQ, Yu J, Luo XZ, Shi YL, Wang Y, Wang L, et al. The high level of RANTES in the ectopic milieu recruits macrophages and induces their tolerance in progression of endometriosis. J Mol Endocrinol. 2010;45:291–299. doi: 10.1677/JME-09-0177. [DOI] [PubMed] [Google Scholar]
- 32.Lebovic DI, Chao VA, Martini JF, Taylor RN. IL-1beta induction of RANTES (regulated upon activation, normal T cell expressed and secreted) chemokine gene expression in endometriotic stromal cells depends on a nuclear factor-kappaB site in the proximal promoter. J Clin Endocrinol Metab. 2001;86:4759–4764. doi: 10.1210/jcem.86.10.7890. [DOI] [PubMed] [Google Scholar]
- 33.May KE, Conduit-Hulbert SA, Villar J, Kirtley S, Kennedy SH, Becker CM. Peripheral biomarkers of endometriosis: a systematic review. Hum Reprod Update. 2010;16:651–674. doi: 10.1093/humupd/dmq009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Borrelli GM, Abrao MS, Mechsner S. Can chemokines be used as biomarkers for endometriosis? A systematic review. Hum Reprod. 2014;29:253–266. doi: 10.1093/humrep/det401. [DOI] [PubMed] [Google Scholar]
- 35.Lawson C, Al-Akoum M, Maheux R, Akoum A. Increased expression of interleukin-1 receptor type 1 in active endometriotic lesions. Reproduction. 2007;133:265–274. doi: 10.1530/rep.1.01121. [DOI] [PubMed] [Google Scholar]
- 36.Kharfi A, Boucher A, Akoum A. Abnormal interleukin-1 receptor type II gene expression in the endometrium of women with endometriosis. Biol Reprod. 2002;66:401–406. doi: 10.1095/biolreprod66.2.401. [DOI] [PubMed] [Google Scholar]
- 37.Tosato G, Jones KD. Interleukin-1 induces interleukin-6 production in peripheral blood monocytes. Blood. 1990;75:1305–1310. [PubMed] [Google Scholar]
- 38.Lin YJ, Lai MD, Lei HY, Wing LY. Neutrophils and macrophages promote angiogenesis in the early stage of endometriosis in a mouse model. Endocrinology. 2006;147:1278–1286. doi: 10.1210/en.2005-0790. [DOI] [PubMed] [Google Scholar]
- 39.Cohen T, Nahari D, Cerem LW, Neufeld G, Levi BZ. Interleukin 6 induces the expression of vascular endothelial growth factor. J Biol Chem. 1996;271:736–741. doi: 10.1074/jbc.271.2.736. [DOI] [PubMed] [Google Scholar]
- 40.Kalu E, Sumar N, Giannopoulos T, Patel P, Croucher C, Sherriff E, et al. Cytokine profiles in serum and peritoneal fluid from infertile women with and without endometriosis. J Obstet Gynaecol Res. 2007;33:490–495. doi: 10.1111/j.1447-0756.2007.00569.x. [DOI] [PubMed] [Google Scholar]
- 41.Bergqvist A, Bruse C, Carlberg M, Carlstrom K. Interleukin 1beta, interleukin-6, and tumor necrosis factor-alpha in endometriotic tissue and in endometrium. Fertil Steril. 2001;75:489–495. doi: 10.1016/s0015-0282(00)01752-0. [DOI] [PubMed] [Google Scholar]
- 42.Chishima F, Hayakawa S, Sugita K, Kinukawa N, Aleemuzzaman S, Nemoto N, et al. Increased expression of cyclooxygenase-2 in local lesions of endometriosis patients. Am J Reprod Immunol. 2002;48:50–56. doi: 10.1034/j.1600-0897.2002.01101.x. [DOI] [PubMed] [Google Scholar]
- 43.Ota H, Igarashi S, Sasaki M, Tanaka T. Distribution of cyclooxygenase-2 in eutopic and ectopic endometrium in endometriosis and adenomyosis. Hum Reprod. 2001;16:561–566. doi: 10.1093/humrep/16.3.561. [DOI] [PubMed] [Google Scholar]
- 44.Kniss DA, Zimmerman PD, Garver CL, Fertel RH. Interleukin-1 receptor antagonist blocks interleukin-1–induced expression of cyclooxygenase-2 in endometrium. Am J Obstet Gynecol. 1997;177:559–567. doi: 10.1016/s0002-9378(97)70146-7. [DOI] [PubMed] [Google Scholar]
- 45.Zhang X, Wen J, Deng L, Lin J. Decreased levels of peritoneal interleukin-1 receptor antagonist in patients with endometriosis and disease-related dysmenorrhea. Fertil Steril. 2007;88:594–599. doi: 10.1016/j.fertnstert.2006.11.155. [DOI] [PubMed] [Google Scholar]
- 46.Kouro T, Takatsu K. IL-5– and eosinophil-mediated inflammation: from discovery to therapy. Int Immunol. 2009;21:1303–1309. doi: 10.1093/intimm/dxp102. [DOI] [PubMed] [Google Scholar]
- 47.Yang Y, Xiao X, Li F, Du L, Kijlstra A, Yang P. Increased IL-7 expression in Vogt-Koyanagi-Harada disease. Invest Ophthalmol Vis Sci. 2012;53:1012–1017. doi: 10.1167/iovs.11-8505. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.