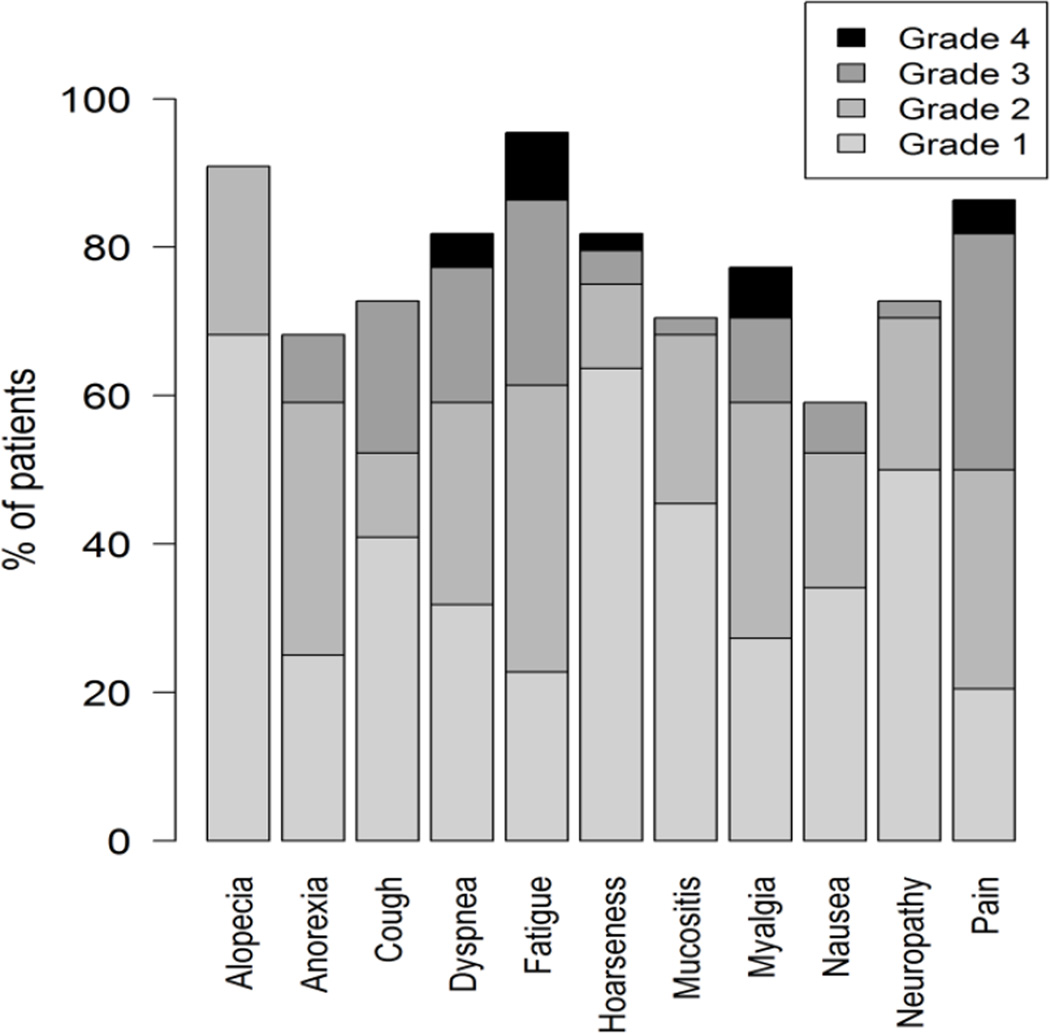
Figure 1.
Example graphical display showing the distribution of maximum post-baseline grades reported by patients during treatment in a phase 2 cancer clinical trial (Clin Trials. 2015 Nov 4. pii: 1740774515615540. PMID:26542025). Grades are based on items modified for patient use from the Common Terminology Criteria for Adverse Events (CTCAE).