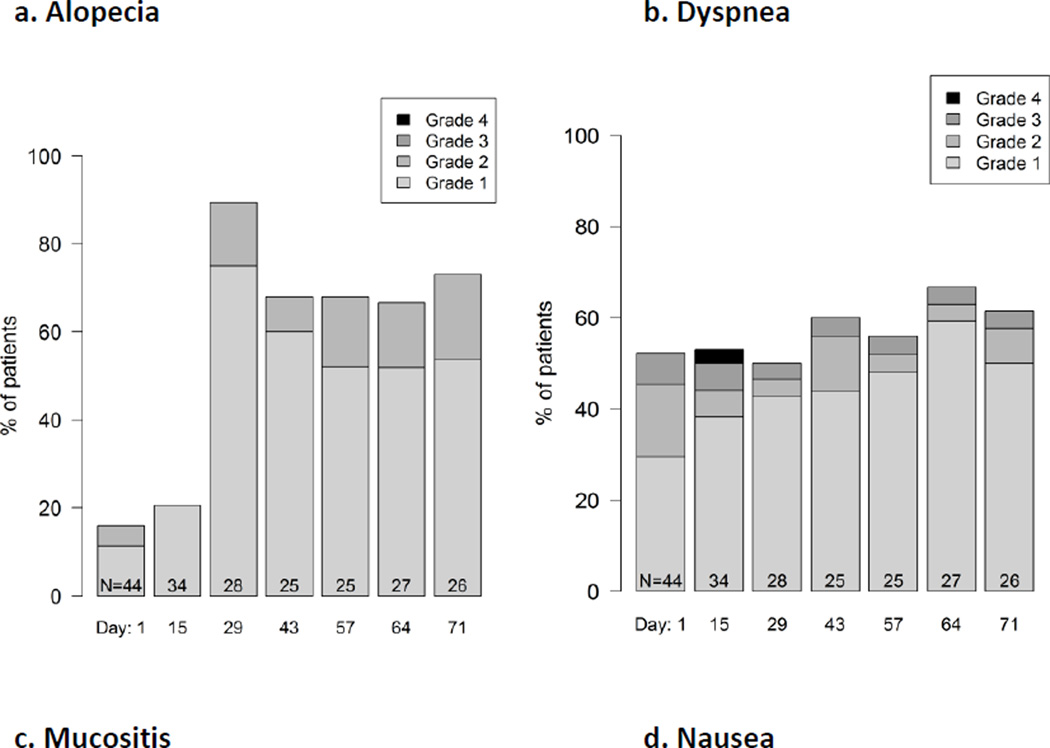
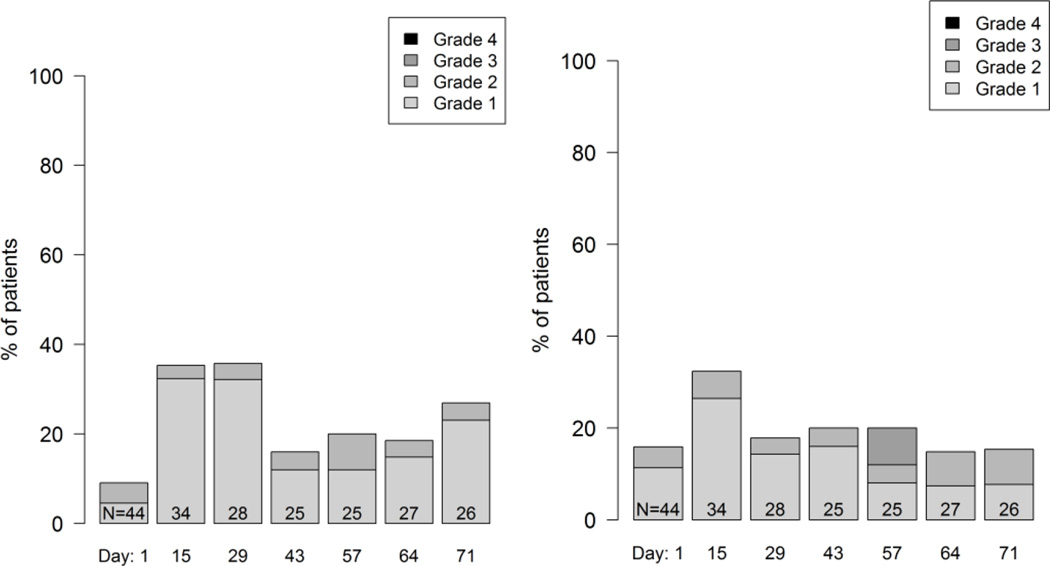
Figure 2.
Example longitudinal graphical displays showing the distribution of patient grade levels for four symptomatic adverse events at time points during treatment in a phase 2 cancer clinical trial (Clin Trials. 2015 Nov 4. pii: 1740774515615540. PMID:26542025). Grades are based on items modified for patient use from the Common Terminology Criteria for Adverse Events (CTCAE). Few patients experienced alopecia at baseline (Day 1) and this number markedly increased at Day 28. In contrast, about half of patients had dyspnea at baseline and this proportion did not change substantially, although the distribution of patient-reported grades improved during the trial, suggesting improvement. Mucositis and nausea both increased at Day 15 then improved, suggesting symptomatic control with supportive measures, and suggests the need for earlier preventive measures for mucositis and nausea with the study regimen.