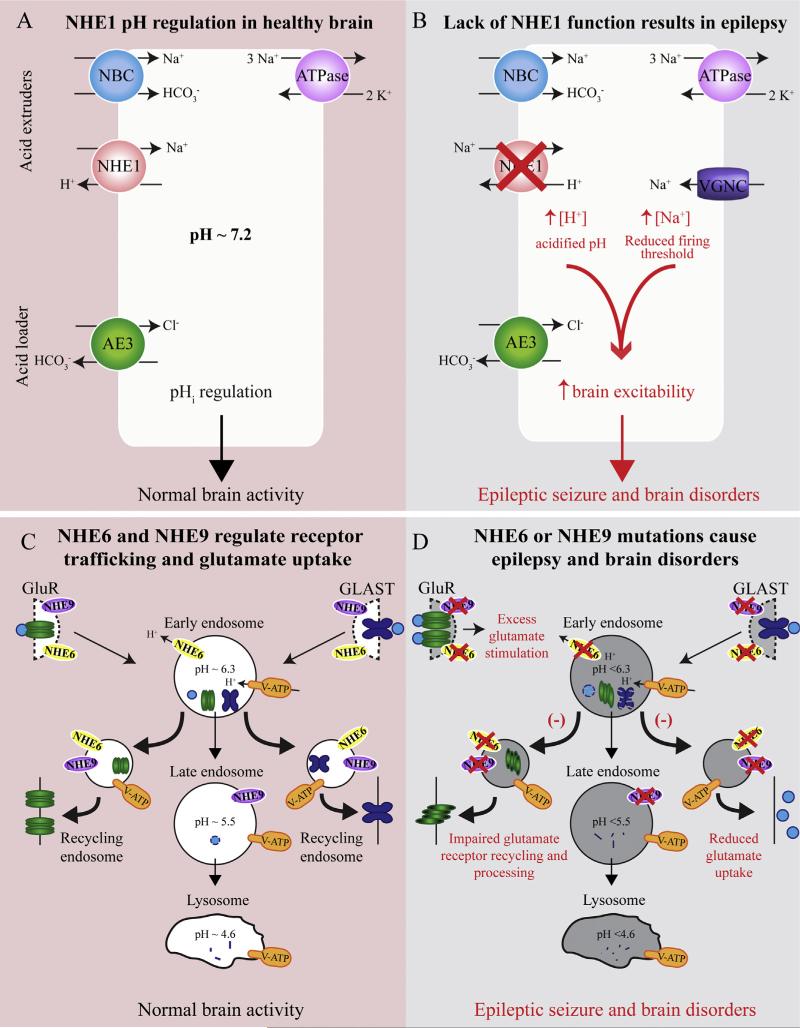
Fig. 3.
Schematic view of impaired functions of NHE1, NHE6, and NHE9 in brain disorders. A cell's pH is regulated by the balance of acid extruders, NHE1 and the sodium coupled bicarbonate transporter (NBC), and the primary acid loader the chloride-bicarbonate exchanger AE3. The sodium-potassium ATPase removes excess Na+ in order to balance ion concentrations. Proper function of all these components is crucial for pHi regulation and normal brain activity.
Mutations that cause NHE1 loss of function result in an acidified intracellular compartment. NHE1 deletion also results in increased expression of voltage sensitive Na+ channels (VGNC), which increases intracellular Na+ concentration and lowers the action potential firing threshold. Together, these ionic changes increase neuronal excitability and facilitate epileptic activity. Intracellular organellar NHE6 and NHE9 are located in early and late endosomes, respectively. Vesicular ATPases (V-ATP)-mediated H+ influx and the NHEs-mediated H+ leakage from the organelles maintain the optimal pH homeostasis between the cytosol, endosome, and lysosome. Glutamate receptors (GluR) and glutamate are endocytosed in endosomes and are either degraded or returned to the membrane. Glutamate transporters, such as GLAST, are also recycled using NHE9 dependent mechanisms. Thus, appropriate pHi regulates protein trafficking, glutamate uptake, and normal brain function. When NHE6 or NHE9 are nonfunctional, the hyperacidified endosomes inhibit appropriate protein processing mechanisms, meaning receptors that are reinserted into the membrane may be misfolded or mislocalized. NHE9 mutations decrease surface expression of GLAST, resulting from hyperacidified endosomes and degradation of the transporter before it can be recycled to the membrane. Decreased GLAST expression will increase extracellular glutamate concentrations, exacerbating the glutamate receptor over stimulation which may lead to epilepsy. Disruption of membrane trafficking and glutamate signaling during development likely contributes to the epilepsy, and other brain disorders including autistic behavior, intellectual disability, ataxia, and attention-deficit/hyperactivity disorder seen in patients with NHE family mutations.