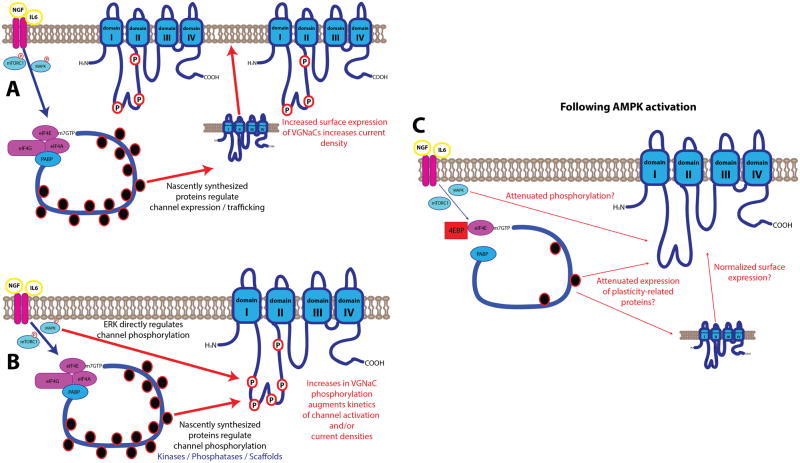
Figure 2. MAPK and mTORC1 signaling in regulation of neuronal excitability and the consequences of AMPK activation.
A) Factors associated with pathological pain such as NGF and IL6 signal to the translation machinery via mTORC1 and MAPK and this is linked to increases in the excitability of DRG and TG neurons. One possible mechanism is that nascently synthesized proteins are involved in the trafficking of channels to the membrane of these neurons. This could lead to enhanced surface expression of these channels, for instance, increased trafficking of voltage gated sodium channels (VGNaCs). B) An alternative mechanism is that signaling via these pathways regulates factors associated with the phosphorylation of channels, thereby increasing DRG and TG neuron excitability. Here MAPK signaling itself can increase channel phosphorylation, including at VGNaCs, and nascently synthesized proteins may also play an important role in regulating channel phosphorylation either via the increased synthesis of kinases involved in these phosphorylation events or via enhanced expression of scaffolding proteins that facilitate channel phosphorylation. C) AMPK activation inhibits mTORC1 and MAPK signaling and would therefore be expected to reduce nascent synthesis of plasticity-related proteins and dampen phosphorylation events downstream of MAPK signaling. This results in decreased DRG and TG neuron excitability. While the precise mechanisms are not known, this is likely linked to channel trafficking or channel phosphorylation or both and VGNaCs may be primary targets for the positive actions of mTORC1 and MAPK signaling and their negative regulation by AMPK.