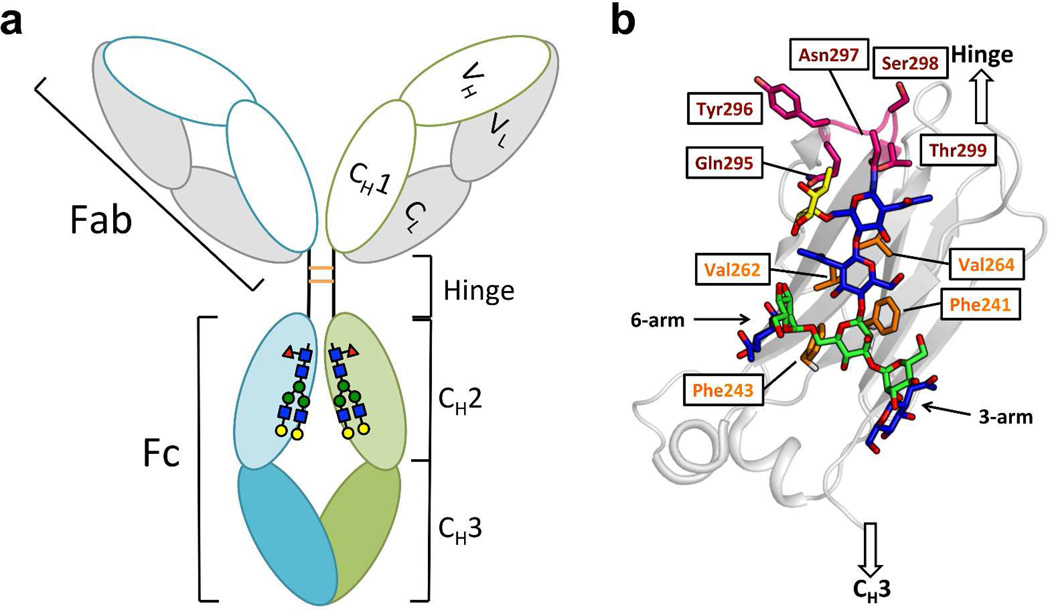
Figure 1. Domain organization and glycosylation site of IgG antibodies.
(a) IgG is composed of the Fab region, the hinge region and the Fc region. The Fab comprises the light chain (grey) and VH and CH1 domains (white) of the heavy chain. The Fc region comprises the CH2 (light green and light cyan) and CH3 (green and cyan) domains of the heavy chain. Each CH2 domain is N-glycosylated at Asn297. The heavy chains are disulfide linked (orange) in the hinge region. (b) Details of the glycan-protein interactions in the CH2 domain (grey ribbon). The N-glycan is presented with GlcNAc colored in blue, mannose colored in green and the core fucose colored in yellow. The N-glycan interacts with the hydrophobic residues (side chains highlighted in orange) on the inner surface of each CH2 domain. The C’E loop (backbone and side chains) is colored in magenta.