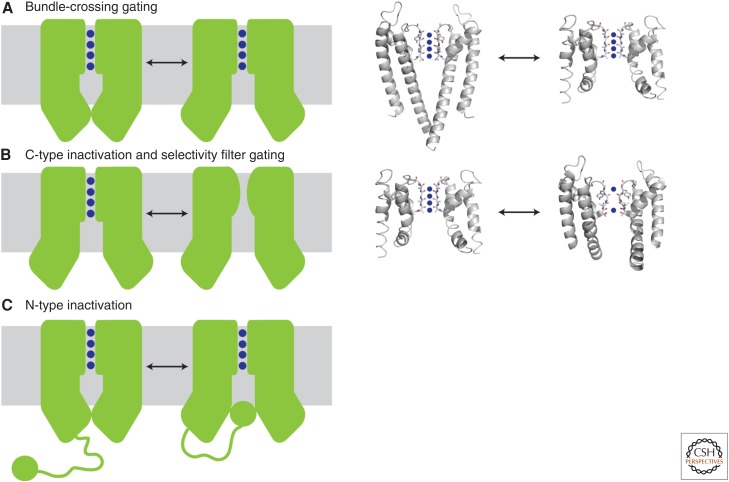
Figure 2.
Gating mechanisms of potassium channels. (A) Gating can occur via conformational changes at the bundle crossing. The inner helices physically block the entry of potassium ions into the aqueous cavity, as shown in the KcsA closed structure (left structure, 1K4C). On activation, the inner helices splay open as observed in MthK (right structure, 1LNQ) and expose the cavity. Blue spheres denote potassium ions. (B) C-type inactivation and selectivity filter gating. In this model, partial collapse of the filter prevents conduction of potassium ions through the pore even if the bundle-crossing gate is open. This model is based on the comparison of the open MthK structure (left structure, 1LNQ) to the collapsed filter of a mutant KcsA in the open state (right structure, 3F5W). (C) N-type inactivation, or ball-and-chain gating, results from binding of an auto-inhibitory peptide to the bundle-crossing gate to physically block entry of ions into the cavity. This peptide can be part of the amino terminus of the channel (as shown) or the amino terminus of an associated β subunit. Currently, no crystal structures exist to illustrate N-type inactivation. The left panels show a cartoon representation of the gating mechanism, and the right panels show the available crystal structures that represent these conformational states.