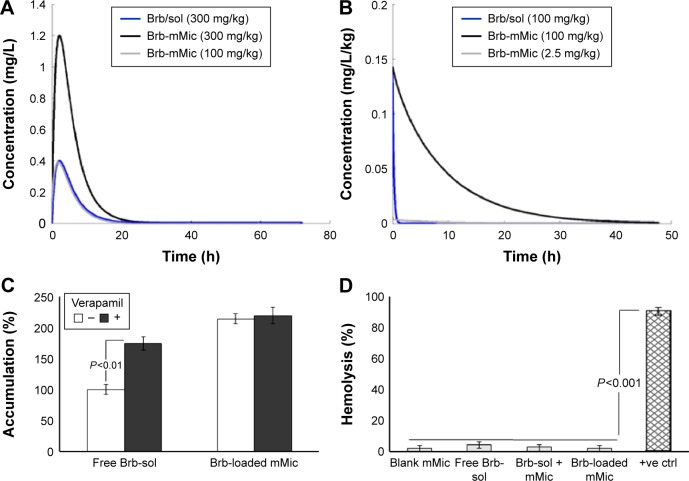
Figure 4.
Simulated pharmacokinetic profiles, colonic cell accumulation, and hemocompatibility of berberine solution and micellar formulation.
Notes: Linear PK simulated at two different dose levels, administered systemically both as (A) human single oral administration of 300 and 100 mg; (B) canine IV bolus infusions of 100 and 2.5 mg. The corresponding open compartmental analysis model parameters were applied accordingly;19,40 (C) quantitative Caco2 cell-based assay of Pgp inhibition, showing drug accumulation in the absence (−) and in the presence (+) of 100 μM of Pgp inhibitor, verapamil hydrochloride, over 3 hours; (D) hemolysis test, after incubation of samples (equivalent to 10 μg/mL of berberine) with red blood cells for 3 hours, 37°C (n=3, mean ± SE).
Abbreviations: Brb, berberine; h, hours; mMic, mixed micelle; PK, photokinetic; IV, intravenous; Pgp, P-glycoprotein; SE, standard error.