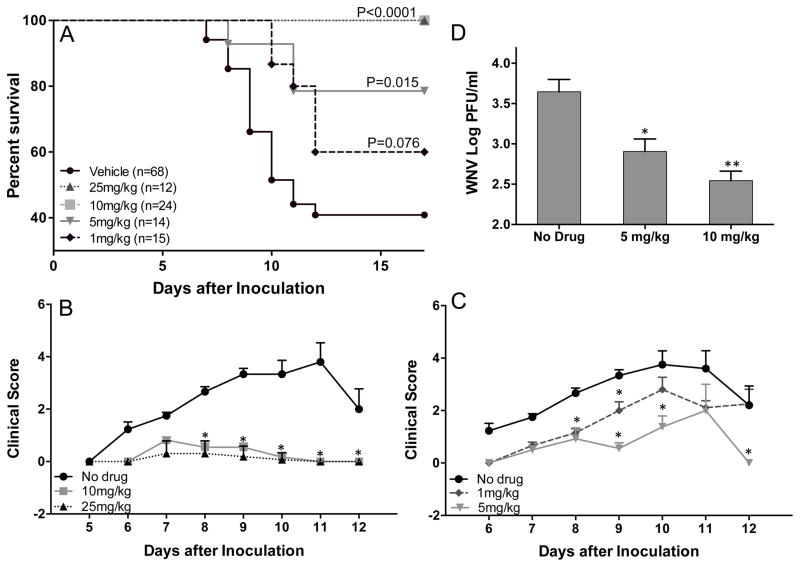
Figure 1.
NITD008 protects against lethal challenge with WNV. (A–C) C57BL/6 mice inoculated with 1000 PFU WNV (NY99) via footpad injection were administered with different doses of NITD008 via oral gavage twice per day from day 1 to 6 after inoculation and observed for clinical symptoms and mortality. Clinical score criteria: 1, ruffled fur; 2, ruffled fur with distinct hunched back; 3, paresis/difficulty walking; 4, paralysis; 5, moribund/euthanized; (D) WNV titers measured in the serum at day 3 after infection using plaque assay. Error bar represent ± SEM from at least 4 mice/group. **p< 0.005, *p<0.05