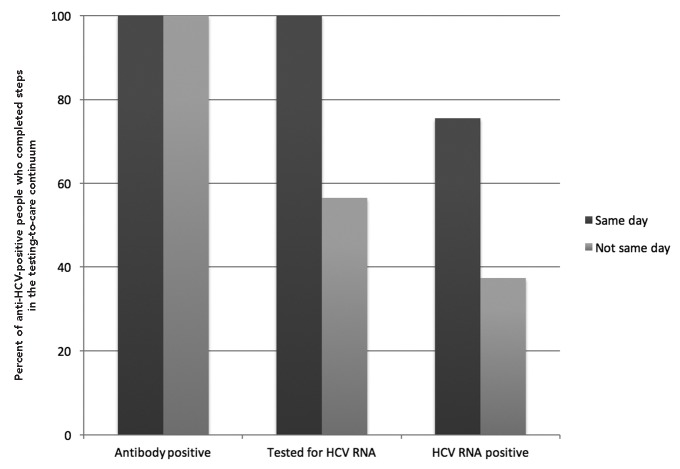
Figure 3.
Proportion of anti-HCV-positive people who were tested for HCV RNA and tested HCV RNA positive, by type of HCV RNA testing (n=2,893), in the Hepatitis Testing and Linkage to Care (HepTLC) initiative, 21 U.S. municipalities, 2012–2014a–d
aThe HepTLC initiative promoted viral hepatitis B and hepatitis C screening, posttest counseling, and linkage to care at 34 U.S. sites. Hepatitis C testing sites were located in San Diego, California; Denver, Colorado; Atlanta, Georgia; Bronx, Ellenville, Rochester, and Queens, New York; Durham, North Carolina; Philadelphia, Pennsylvania; Aguas Buenas, Caguas, and San Juan, Yabucoa, Puerto Rico; Bamberg, Cheraw, Fairfax, Florence, Mullens, Orangeburg, South Carolina; San Antonio, Texas; and Washington, D.C.
bSeven people without a valid antibody test date were excluded.
cAntibody positive for same-day testing included 1,088 participants.
dAntibody positive for non-same-day testing included 1,805 participants.
anti-HCV = hepatitis C virus antibody
HCV = hepatitis C virus
RNA = ribonucleic acid