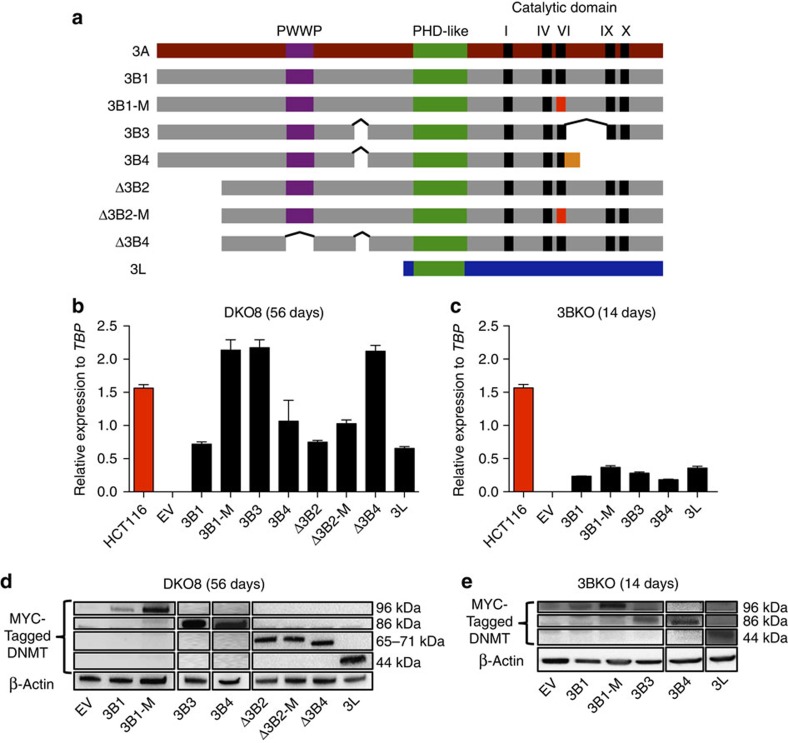
Figure 1. Stable reintroductions of DNMT3B isoforms and DNMT3L in DKO8 and 3BKO cell lines.
(a) Schematic diagram of DNMT3A (3A), DNMT3B isoforms (3B1, 3B1-M, 3B3, 3B4, Δ3B2, Δ3B2-M and Δ3B4) and DNMT3L (3L) showing conserved PWWP (purple), PHD-like domain (green) and DNMT catalytic motifs (black). There are five catalytic domains (I, IV, VI, IX and X) in DNMT3A and DNMT3B, all of which are absent in DNMT3L. A red-coloured VI domain indicates inactivating mutations (Cys to Ser) of amino acids 651 and 452 in 3B1-M and Δ3B2-M, respectively. 3B4 has a frameshift with a unique protein sequence shown in orange. ^indicates alternative splicing. (b,c) mRNA expression level of endogenous DNMT3B in HCT116 cells, exogenous DNMT3B isoforms and DNMT3L assessed by qRT-PCR and normalized to the expression of the TATA Box Binding Protein (TBP) in DKO8 cells, 56 days post transfection and 3BKO cells, 14 days post transfection, respectively. Error bars indicate standard deviation from the mean of three biological replicates. The empty vector (EV) cell line is the transfection control. (d,e) Protein-expression levels of exogenous MYC-tagged DNMT isoforms by western blot analysis in DKO8 cells, 56 days post transfection and 3BKO cells, 14 days post transfection, respectively. β-ACTIN was used as a loading control.