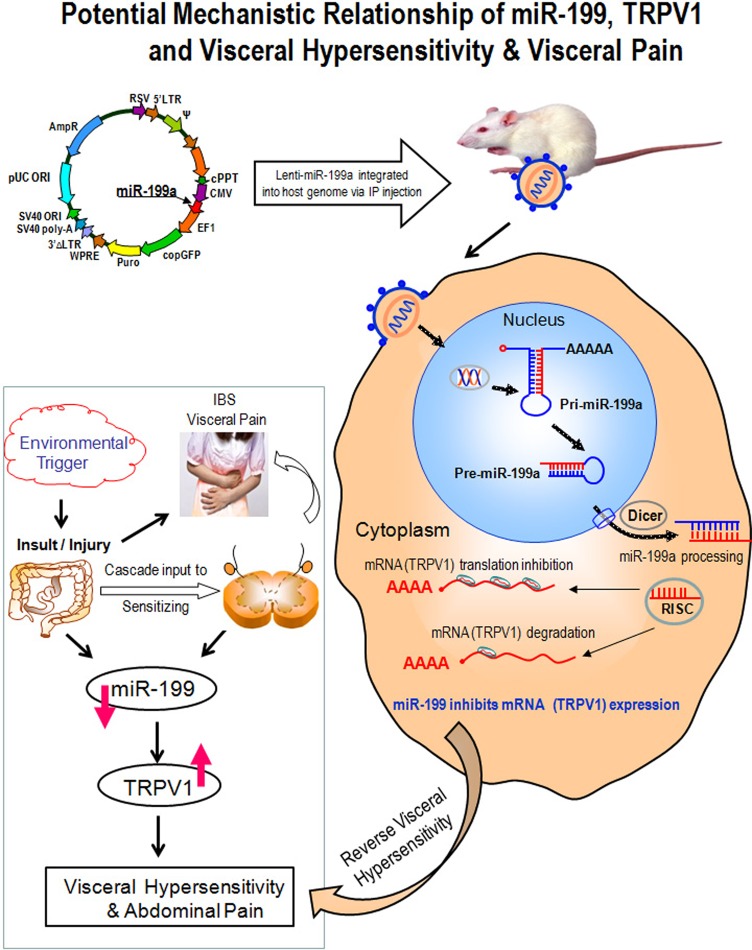
Figure 5.
Potential mechanistic relationship of miR-199, transient receptor potential vanilloid type 1 (TRPV1) and visceral hypersensitivity and visceral pain. Lentiviruses deliver microRNAs (miRNAs) in vivo and offer several potentially unique advantages over conventional retroviral gene delivery systems. Principal among these is the ability to provide a long-term and stable gene/miRNA expression and to infect non-dividing cells, such as dorsal root ganglion neuron cells. This study used lentiviruses to deliver and overexpress miR-199a to silence the TRPV1 expression. The related molecular mechanisms are illustrated. Left lower panel: environmental triggers lead to gut injury, which sets up a cascade of events that leads to a state of visceral hypersensitivity. This hypersensitivity state may be characterised by decreased miR-199 and increased TRPV1 expression. Right: after a host cell is infected by a RNA virus, the viral genome integrated into host genome in the nucleus to yield pri-miR-199a. The pri-miR-199a is processed by host factors in the nucleus to yield the pre-miRNA intermediate, which is then exported to the cytoplasm through exportin, where the mature miRNA (miR-199a) is generated via Dicer involvement and incorporated into the RISCs that are programmed by miRNA, and then inhibit expression of the host mRNAs (TRPV1) in the infected cell's cytoplasm. Decreased TRPV1 expression reverses visceral hypersensitivity. RISC, RNA-induced silencing complex.