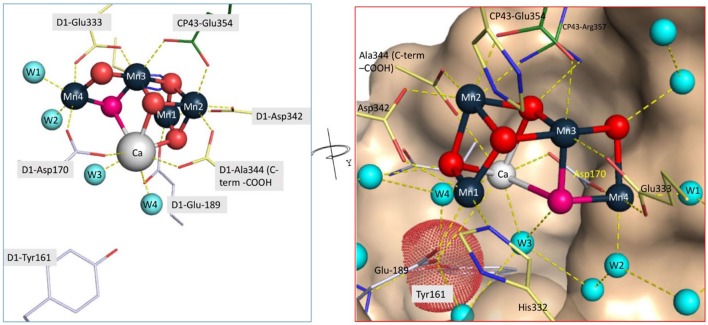
Figure 2.
Coordination environment of the assembled Mn4CaO5 H2O-oxidation complex of PSII. The high affinity site of Mn2+ binding and photooxidation during the initial phase of the assembly process minimally involves D1-Asparate170 (Nixon and Diner, 1992; Campbell et al., 2000) located in the vicinity of Mn4 in the final complex. The initial state of the complex for photoassembly appears to involve the binding of one Mn2+ at the high affinity site (Ono and Mino, 1999) together with one Ca2+ ion that modulates the ligand environment of the Mn2+ possibly via the formation of a bridging water or hydroxide, although the presence of the Ca2+ does not appreciably change the binding affinity of the Mn2+ at the high affinity site (Tyryshkin et al., 2006). The C-terminal polypeptide backbone carboxylate of D1-Alanine344, which is available only following proteolytic cleavage of the precursor form of the D1 protein (pD1), is also critical for the assembly process, although it too does not markedly alter the binding of Mn2+ at the high affinity site (Nixon et al., 1992; Cohen et al., 2007). Figures developed upon 3D coordinates (PDB 4UB6) of the published X-ray diffraction model (Umena et al., 2011).