Fig. 1. Link systemically, act locally.
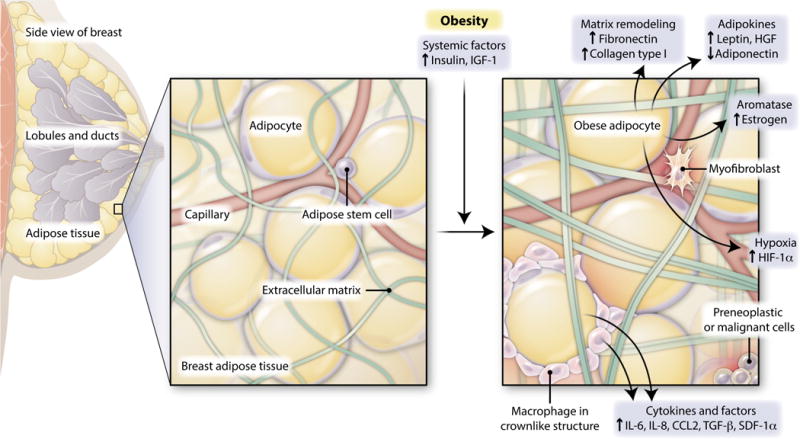
Obesity induces both local and systemic changes implicated in breast cancer progression. Obesity-induced systemic changes include increases in circulating insulin and insulin-like growth factor–1 (IGF-1), which can act within the breast microenvironment to promote cancer progression. Adipocytes in the breast might contribute to breast tumor development through the increased secretion of adipocyte-derived hormones and growth factors (adipokines), including the hormone leptin and paracrine growth factor hepatocyte growth factor (HGF), and the diminished secretion of adiponectin, which is a protein involved in the regulation of glucose levels. Adipocytes also produce aromatase, which can lead to increased local estrogen concentrations in the obese breast. Adipocytes or recruited immune cells (including macrophages), which form characteristic crownlike structures around adipocytes, secrete increased amounts of multiple cytokines and other growth factors in breast adipose depot. Unregulated growth of adipocytes under conditions of obesity also produces hypoxic conditions, which may elevate localized angiogenesis. Now, Seo et al. have identified an additional mechanism by which increased collagen and fibronectin deposition within obese adipose tissue contributes to ECM stiffness, which promotes breast tumor progression.
