Abstract
Background
Chronic cluster headache (CH) is a debilitating disorder for which few well-controlled studies demonstrate effectiveness of available therapies. Non-invasive vagus nerve stimulation (nVNS) was examined as adjunctive prophylactic treatment of chronic CH.
Methods
PREVA was a prospective, open-label, randomised study that compared adjunctive prophylactic nVNS (n = 48) with standard of care (SoC) alone (control (n = 49)). A two-week baseline phase was followed by a four-week randomised phase (SoC plus nVNS vs control) and a four-week extension phase (SoC plus nVNS). The primary end point was the reduction in the mean number of CH attacks per week. Response rate, abortive medication use and safety/tolerability were also assessed.
Results
During the randomised phase, individuals in the intent-to-treat population treated with SoC plus nVNS (n = 45) had a significantly greater reduction in the number of attacks per week vs controls (n = 48) (−5.9 vs −2.1, respectively) for a mean therapeutic gain of 3.9 fewer attacks per week (95% CI: 0.5, 7.2; p = 0.02). Higher ≥50% response rates were also observed with SoC plus nVNS (40% (18/45)) vs controls (8.3% (4/48); p < 0.001). No serious treatment-related adverse events occurred.
Conclusion
Adjunctive prophylactic nVNS is a well-tolerated novel treatment for chronic CH, offering clinical benefits beyond those with SoC.
Keywords: Neuromodulation, cluster headache, adjunctive prophylaxis, vagus nerve
Introduction
Cluster headache (CH) is a trigeminal autonomic cephalalgia with an estimated lifetime prevalence of 124 per 100,000 adults (1). CH attacks are unilateral, with severe pain located in periorbital, retro-orbital and temporal regions. Pain peaks within minutes, may last up to three hours if untreated (2,3) and is usually accompanied by cranial autonomic symptoms (e.g. conjunctival injection or lacrimation, nasal congestion or rhinorrhoea, periorbital oedema) and agitation (1,2). If attacks occur for >1 year without pain-free remission lasting ≥1 month, CH is classified as chronic (3).
Abortive therapy aims to reduce the severity and duration of CH attacks (4). First-line abortive treatments include inhaled oxygen (2,5) and subcutaneous (SC) or intranasal triptans (2,4,6). Verapamil is a mainstay of prophylactic therapy; however, it is not approved for this indication, and only two randomised controlled trials with level-C evidence support its use (4). Although other pharmacologic interventions (e.g. lithium, oral steroids, valproic acid, topiramate, ergotamine, suboccipital steroid injections) have been investigated, most lack compelling evidence to support their use (2,4,7); suboccipital steroid injections have demonstrated efficacy as adjunctive prophylactic therapy for CH in patients receiving verapamil (8).
Vagus nerve stimulation (VNS) has been investigated as adjunctive or first-line treatment for CH (9). The strong parasympathetic activation that occurs during CH attacks (10) supports the rationale for nVNS therapy. Early data suggested that inhibition of pain by VNS occurs by inhibition of vagal afferents to the trigeminal nucleus caudalis (TNC) (11). More recent evidence suggests that VNS may also inhibit pain by modulating inhibitory neurotransmitter release, leading to decreased TNC glutamate levels (12,13). The Prevention and Acute Treatment of Chronic Cluster Headache (PREVA) trial is the first randomised controlled study that examined non-invasive VNS (nVNS; gammaCore®) as adjunctive prophylactic therapy for CH attacks in patients with chronic CH.
Methods
Study design
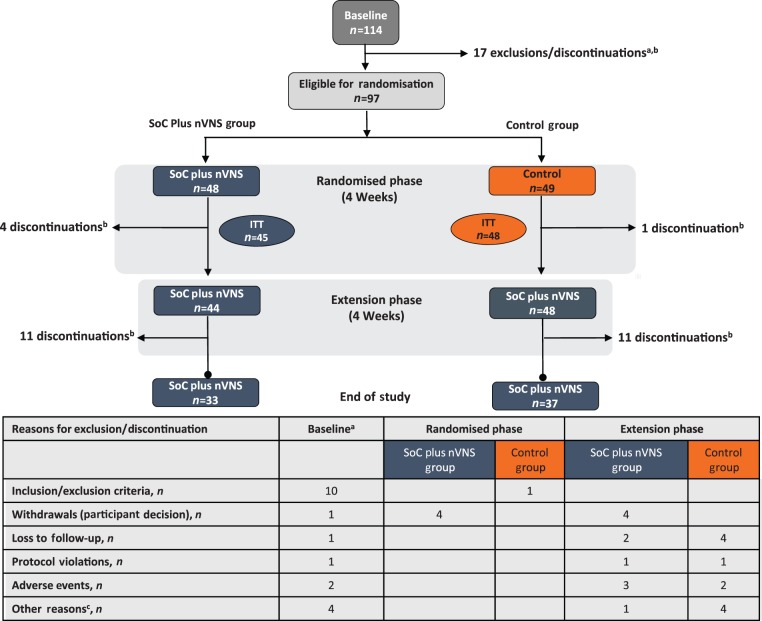
This prospective, multicentre, open-label, randomised, controlled, parallel-group study, conducted from October 2012 through March 2014, involved 10 European sites: five in Germany, three in the United Kingdom, one in Belgium, and one in Italy. The study was funded by electroCore, LLC, and was conducted in accordance with principles and requirements of the Declaration of Helsinki, Good Clinical Practices and clinical trial registration (ClinicalTrials.gov Identifier NCT01701245). All investigators obtained institutional review board approval, and all participants provided written informed consent. The study comprised a two-week baseline phase during which all participants received only their individualised standard of care (SoC); a four-week randomised phase during which participants were randomly assigned 1:1 by standard block design to receive either SoC plus nVNS or SoC alone (control); and an optional four-week extension phase during which all participants received SoC plus nVNS (Figure 1).
Figure 1.
Participant disposition.
ITT: intent-to-treat; mITT: modified intent-to-treat; nVNS: non-invasive vagus nerve stimulation; SoC: standard of care.
aExclusions or discontinuations for more than one reason occurred in some individuals.
bRefer to the table below the figure for a breakdown of discontinuations by reason.
cOther reasons for discontinuation included inability to fulfil visits because of injury, inability to continue the study because of family commitments, dissatisfaction with or discontinued use of the device, and noncompliance with study procedures.
Study population
Participants were aged 18 to 70 years and were diagnosed with chronic CH according to International Classification of Headache Disorders criteria (3) ≥1 year before enrolment. Key exclusion criteria were change in prophylactic medication type or dosage <1 month before enrolment; history of intracranial/carotid aneurysm or haemorrhage; brain tumours/lesions; significant head trauma; previous surgery or abnormal anatomy at the nVNS treatment site; known or suspected cardiac/cardiovascular disease; implantation with electrical or neurostimulation devices; history of carotid endarterectomy or vascular neck surgery; implantation with metallic hardware; and recent history of syncope or seizures.
Intervention
The nVNS device produced a low-voltage electrical signal (5-kHz sine wave series that occurred for 1 ms and repeated every 40 ms (25 Hz)). When applied against the neck, the device delivered a maximum of 24 V and 60 mA output current while allowing stimulation amplitude adjustment by the user. Two stainless steel contact surfaces and conductive gel applied by the user enabled delivery of stimulations. Mandatory nVNS prophylaxis consisted of three 2-minute stimulations (i.e. three doses) five minutes apart administered twice daily (i.e. six doses per day) to the right side of the neck (right vagal nerve). The first prophylactic treatment was administered within one hour of waking; the second was administered seven to 10 hours after the first treatment. The number, duration, frequency, and timing of doses were chosen according to previously reported nVNS dosing parameters (9). Participants also had the option of acutely treating CH attacks with three additional nVNS doses at pain onset but were advised to not administer prophylactic therapy within a two-hour period after acute treatment. If the CH attack was not aborted within 15 minutes after stimulation, individuals were instructed to take abortive medications (e.g. SC sumatriptan, inhaled oxygen and intranasal zolmitriptan). Changes in SoC prophylactic medications (e.g. verapamil, lithium, topiramate and corticosteroids) were not permitted throughout the study.
Objectives, efficacy assessments and end points
The objective was to assess the efficacy of adjunctive prophylactic nVNS therapy in chronic CH. The primary end point was the reduction in the mean number of CH attacks per week, defined as the number of attacks during the last two weeks of the randomised phase minus the number of attacks during baseline divided by 2. Attack frequency was evaluated during the last two weeks of the four-week randomised phase to ensure sufficient time for nVNS to demonstrate its full effect. Reductions in the mean number of CH attacks per week were also evaluated during the last two weeks of the extension phase.
Secondary efficacy end points included ≥50% response rate (i.e. proportion of participants with ≥50% reduction in mean number of CH attacks per week), abortive medication use and duration and intensity of CH attacks that were acutely treated with nVNS. The ≥50% response rate was assessed during the last two weeks of the randomised phase and the last two weeks of the extension phase. All other secondary end points were assessed at baseline and during the last two weeks of the randomised and extension phases. Participant-completed headache diaries captured the number of CH attacks, CH pain intensity (five-point scale: none to very severe), CH duration and abortive medication use. The EQ-5D-3L (14) and six-item Headache Impact Test (HIT-6™) (15) instruments were used to assess quality of life (QoL) at the end of baseline and at the end of both treatment phases. Adherence to nVNS treatment was evaluated in each phase by dividing the actual number of doses administered by the prescribed number of doses.
Safety, tolerability and perceptions of nVNS
Safety and tolerability were assessed by evaluating adverse events (AEs), vital signs and physical examination results. Participants recorded AEs in their headache diaries; additional AE information and vital sign/physical examination results were obtained by the clinical investigator at scheduled clinic visits. AEs were summarised by frequency, treatment relatedness and severity. Satisfaction with nVNS (five-point scale: extremely satisfied to not at all satisfied), recommendation of the device to a family member or friend (yes/no), and ease of device use (four-point scale: very easy to very difficult) were evaluated at the end of the extension phase.
Sample size determination
Enrolment of 90 individuals was planned, with an estimated dropout rate of 10%. The mean frequency of CH attacks at baseline was assumed to be 4.0 per week. Predicted reductions in the number of CH attacks per week were 50% for the SoC plus nVNS group and 10% for the control group. A sample size of 40 participants per treatment arm had 80% power to detect between-group differences in mean change from baseline using a two-sided test with α ≤ 0.05. An interim analysis of sample size was performed after enrolment of 30 people in each treatment group. Mean reductions in the number of CH attacks per week for SoC plus nVNS and control arms were 5.5 and 1.1, respectively (common standard deviation (SD), 6.87); the effect size was 0.65.
Statistical analyses
Safety and tolerability were assessed in the safety population (i.e. all individuals assigned to treatment). The intent-to-treat (ITT) population was defined as all participants who had ≥1 efficacy recording in the headache diary after randomisation. The modified ITT (mITT) population was defined as participants who had measurable observations across the respective study phases being compared (i.e. baseline vs randomised phase, baseline vs extension phase or randomised vs extension phase). The reduction in the number of CH attacks per week from baseline to the randomised phase (primary end point) was assessed in the ITT population, for which missing data were imputed to 0 (i.e. no change; designated as treatment failures). The change in the number of CH attacks from the randomised phase to the extension phase was assessed in the mITT population. The ≥50% response rate was assessed in the ITT (with imputation to no response for missing data) and mITT populations. Because no formal hypotheses were associated with secondary outcomes (i.e. abortive medication use, QoL), these data were assessed in the mITT population.
Analysis of variance and analysis of covariance (site as covariate) were used to assess differences between treatment groups for the primary end point and the change in duration and intensity of CH attacks. Within-participant differences in the number of CH attacks and pain intensity ratings reported during the randomised and extension phases were analysed using the Wilcoxon rank sum test. Differences in response rates between treatment groups were evaluated using chi-square analysis without continuity correction. Two-sided p values were calculated, and p < 0.05 was considered statistically significant.
Results
Participant disposition, demographics and treatment adherence
Of the 114 individuals enrolled and assessed at baseline, 97 were randomly assigned to treatment and constituted the safety population (SoC plus nVNS, n = 48; control, n = 49); 92 continued into the extension phase (SoC plus nVNS, n = 44; control, n = 48); and 70 completed the study (SoC plus nVNS, n = 33; control, n = 37) (Figure 1). Of the 97 individuals in the safety population, 93 met the criteria for inclusion in the ITT population (SoC plus nVNS, n = 45; control, n = 48). Because the mITT population relied on the availability of measurable observations across the study phases being compared, the number of participants who met the criteria for inclusion in this population varied among the individual end points.
Demographics and baseline characteristics were similar between groups and were representative of the overall CH population (2); use of SoC prophylactic medications was also comparable between groups (Table 1). In the ITT population, 64.4% (29/45) of individuals assigned to SoC plus nVNS were ≥80% adherent to nVNS treatment during the randomised and extension phases; 50% (24/48) of participants assigned to control were ≥80% adherent to nVNS treatment during the extension phase.
Table 1.
Demographics and baseline characteristics (safety population).
| Characteristic | SoC plus nVNS (n = 48) | Control (n = 49) |
|---|---|---|
| Age, y, mean (SD) | 45.4 (11.0) | 42.3 (11.0) |
| Sex, n (%) | ||
| Male | 34 (71) | 33 (67) |
| Time since onset of chronic CH disorder, y, mean (SD)a | 4.7 (3.9) | 5.0 (3.7) |
| CH attack duration, min, mean (SD) | ||
| With acute pharmacologic medications/oxygenb | 27.4 (19.8) | 29.3 (29.9) |
| Without acute pharmacologic medications/oxygenc | 95.2 (57.7) | 103.3 (66.8) |
| Number of CH attacks in the four weeks before enrolment, mean (SD)c | 67.3 (43.6) | 73.9 (115.8) |
| Use of prophylactic medications for CH, n (%) | ||
| Verapamil/verapamil hydrochloride | 25 (52) | 26 (53) |
| Lithium/lithium carbonate | 6 (13) | 9 (18) |
| Topiramate | 7 (15) | 7 (14) |
| Corticosteroids | 2 (4) | 2 (4) |
| Use of pharmacologic medications/oxygen for the acute treatment of CH, n (%) | ||
| Pharmacologic medications | 43 (90) | 44 (90) |
| Oxygen | 32 (67) | 34 (69) |
CH: cluster headache; nVNS: non-invasive vagus nerve stimulation; SD: standard deviation; SoC: standard of care; y: years.
Data were missing for two participants in the control group.
Data were missing for one participant in the control group.
Data were missing for one participant in the SoC plus nVNS group.
Effect of nVNS on CH attack frequency
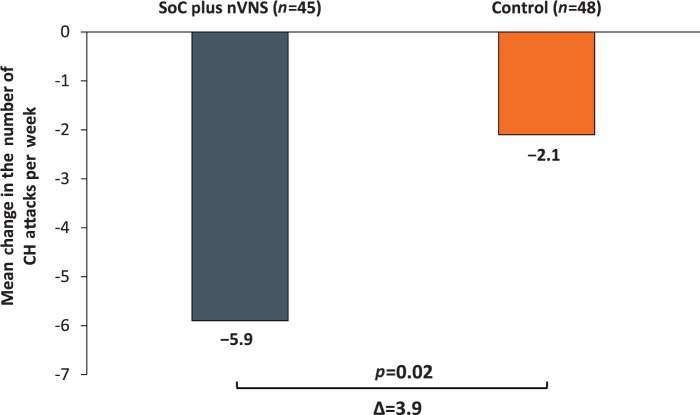
In the ITT population (SoC plus nVNS, n = 45; control, n = 48), participants receiving SoC plus nVNS during the randomised phase had a greater reduction from baseline (−5.9; SE, 1.2) in the number of CH attacks per week than those receiving control (−2.1; SE, 1.2), for a mean therapeutic gain of 3.9 fewer CH attacks per week (95% confidence interval (CI): 0.5, 7.2; p = 0.02) (Figure 2). In the site-adjusted model, the mean therapeutic gain was 4.2 fewer headache attacks per week (95% CI: 0.8, 7.5; p = 0.02).
Figure 2.
Mean change in the number of CH attacks per week: baseline vs the last two weeks of the randomised phase (ITT population).
CH: cluster headache; ITT: intent-to-treat; nVNS: non-invasive vagus nerve stimulation; SoC: standard of care.
Values are presented as unadjusted means and were calculated from all participants with evaluable data.
p value corresponds to the difference in the change from baseline between treatment groups from an analysis of variance.
To determine the efficacy of longer-term prophylactic use of nVNS, the reduction in the number of CH attacks during the extension phase was examined in the mITT population. Individuals who continued using nVNS through the extension phase (n = 30) reported an additional reduction of two CH attacks per week (randomised phase, 9.6; extension phase, 7.6; p < 0.001), suggesting further benefit with continued use. Upon addition of nVNS in the control group (n = 41) during the extension phase, mITT individuals reported a significant reduction of 3.3 CH attacks per week (randomised phase, 15.7; extension phase, 12.4; p < 0.001).
≥50% Response rates
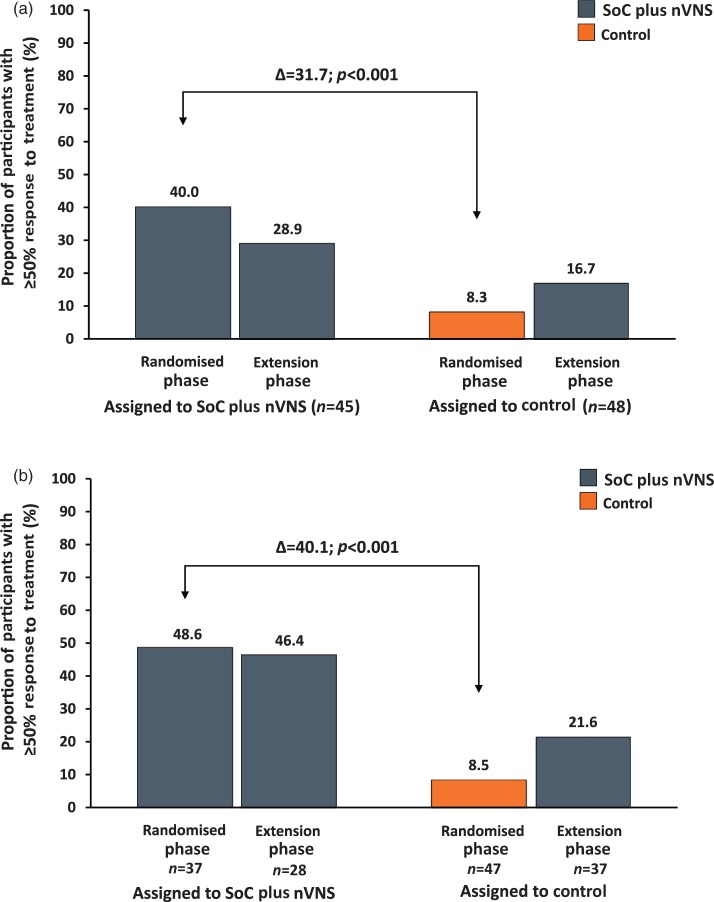
Among participants in the ITT population, a significantly higher ≥ 50% response rate during the randomised phase was observed in the SoC plus nVNS group (40% (18/45)) than in the control group (8.3% (4/48)) (p < 0.001) (Figure 3(a)). Similarly, the response rate in the mITT population (SoC plus nVNS, n = 37; control, n = 47) was also significantly higher for individuals receiving SoC plus nVNS (48.6% (18/37)) than for the control group (8.5% (4/47)) (p < 0.001; Figure 3(b)), suggesting that participants who remained in the study continued to respond. During the extension phase, ≥50% response rates in ITT individuals who continued adjunctive nVNS and those who began nVNS were 28.9% and 16.7%, respectively (Figure 3(a)).
Figure 3.
≥50% Response to treatmenta.
(a) ITT population.
(b) mITT population.
CH: cluster headache; ITT: intent-to-treat; mITT: modified intent-to-treat; nVNS: non-invasive vagus nerve stimulation; SoC: standard of care.
aProportion of individuals with ≥50% reduction in the mean number of CH attacks per week.
Data were calculated at the end of the treatment phases.
p values correspond to the difference in response rate between treatment groups.
The response rate for mITT individuals continuing treatment with nVNS (Figure 3(b)) through the end of the extension phase was 46.4% (13/28). The response rate for mITT individuals in the control arm who participated in the extension phase (n = 37) increased from 8.5% (4/47) during the randomised phase to 21.6% (8/37) with the addition of nVNS in the extension phase.
Abortive medication use
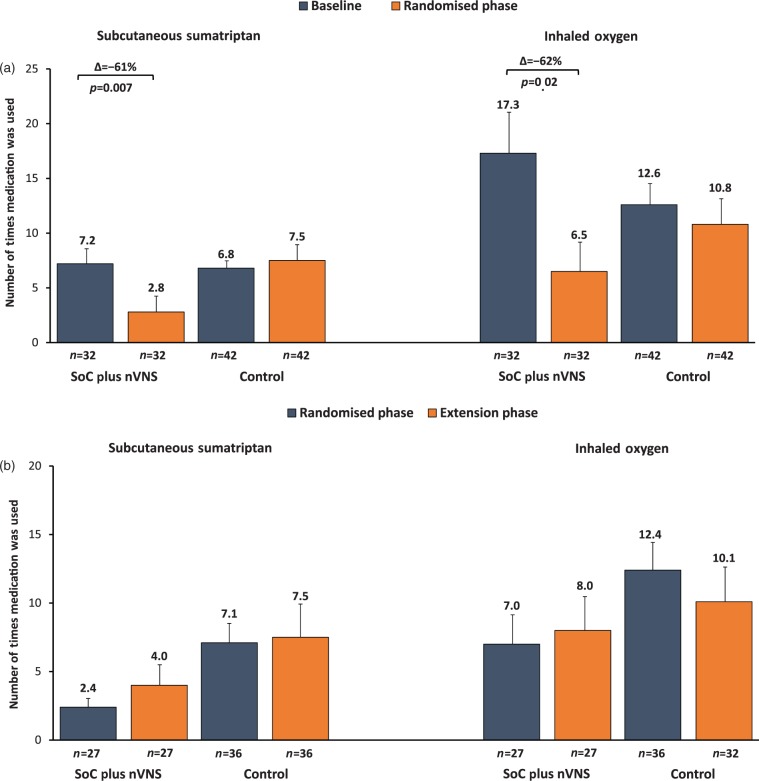
The number of times abortive medications were used in the mITT population during the last two weeks of each study phase is presented in Figure 4. During the randomised phase, a 57% decrease in the frequency of abortive medication use was noted in the SoC plus nVNS group (Δ = −15 (95% CI: −22.8, −7.2); p < 0.001). In contrast, individuals assigned to the control group did not experience a substantial reduction in abortive medication use (Δ = −2 (95% CI: −9.4, 5.4); p = 0.59). Changes in abortive medication use among participants assigned to SoC plus nVNS were driven by >60% reductions in the use of SC sumatriptan (Δ = −4.4 (95% CI: −7.6, −1.2); p = 0.007) and inhaled oxygen (Δ = −10.8 (95% CI: −19.4, −2.2); p = 0.02) (Figure 4(a)); such reductions were maintained during the extension phase (Figure 4(b)). Among individuals previously assigned to the control group, adjunctive prophylactic nVNS during the extension phase did not result in a significant reduction in the use of any abortive medication (Δ = −3.4 (95% CI: −11.5, 4.7); p = 0.40).
Figure 4.
Abortive medication use (mITT population).
(a) Use of sumatriptan and oxygen: baseline vs the last two weeks of the randomised phase.
(b) Use of sumatriptan and oxygen: last two weeks of the randomised phase vs the last two weeks of the extension phase.
mITT: modified intent-to-treat; nVNS: non-invasive vagus nerve stimulation; SoC: standard of care.
Values are presented as means and were calculated from all participants with evaluable data.
Error bars denote standard error of the mean.
Use of nVNS as abortive therapy
During the randomised phase, 93.8% (45/48) of individuals assigned to SoC plus nVNS acutely treated ≥1 CH attack with nVNS; during the extension phase, 68.2% (30/44) and 83.3% (40/48) assigned to SoC plus nVNS and control, respectively, acutely treated ≥1 CH attack with nVNS. The optional use of nVNS as abortive therapy for CH attacks had no effect on CH attack duration or pain intensity (data not shown).
QoL outcomes
Changes from baseline in the mean EQ-5D-3L index score for the mITT population were greater for the SoC plus nVNS group than for the control group (Table 2), indicating a significant improvement with nVNS (SoC plus nVNS minus control: Δ = 0.194 point (95% CI: 0.054, 0.334 point); p = 0.007). The change in EQ-5D-3L index score for participants receiving SoC plus nVNS (0.145 point) was above the reported minimally important difference (MID; 0.074 point) (16) and was considered clinically meaningful. Addition of nVNS in participants previously assigned to the control group was associated with a clinically meaningful change in EQ-5D-3L index score during the extension phase (0.078 point (95% CI: −0.02, 0.18 point)) (Table 2).
Table 2.
Quality of life outcomes (mITT population).
| QoL measures | SoC plus nVNS |
Control |
SoC plus nVNS |
Control |
|---|---|---|---|---|
| Mean change from baseline to randomised phasea,b | Mean change from baseline to extension phasec,d | |||
| EQ-5D-3L Index score | 0.145e | −0.049 | 0.155 | 0.078 |
| EQ-5D-3L VAS score | 9.20f | 0.27 | 10.79 | 4.36 |
| HIT-6 score | −2.78 | −0.47 | −3.28 | −2.77 |
HIT-6: 6-item Headache Impact Test; mITT: modified intent-to-treat; nVNS: non-invasive vagus nerve stimulation; QoL: quality of life; SoC: standard of care; VAS: visual analogue scale.
Number of participants evaluated for EQ-5D-3L index score: SoC plus nVNS, n = 35, and control, n = 46; EQ-5D-3L VAS score: SoC plus nVNS, n = 35, and control, n = 45.
Number of participants evaluated for HIT-6 score: SoC plus nVNS, n = 37, and control, n = 45.
Number of participants evaluated for EQ-5D-3L index score: SoC plus nVNS, n = 34, and control, n = 40; EQ-5D-3L VAS score: SoC plus nVNS, n = 34, and control, n = 39.
Number of participants evaluated for HIT-6 score: SoC plus nVNS, n = 36, and control, n = 39.
p = 0.007 vs control.
p = 0.039 vs control.
In the randomised phase, the change from baseline in the mean EQ-5D-3L visual analogue scale (VAS) score was greater for the SoC plus nVNS group than for the control group (Table 2) and reflected a significant improvement with nVNS (SoC plus nVNS minus control: Δ = 8.93 points (95% CI: 0.47, 17.39 points); p = 0.039). During the extension phase, individuals who were previously assigned to control also showed improved VAS scores (Table 2). Although changes in mean HIT-6 scores in the SoC plus nVNS group were greater than those in the control group (Table 2) and were above the reported MID (−2.3 points) (17), absolute mean HIT-6 scores suggested that CH attacks continued to have a substantial impact on QoL (data not shown).
Safety, tolerability and perceptions of nVNS
A total of seven individuals discontinued participation because of AEs (Table 3); only two AEs (depressed mood and CH) occurred in >1 person. During the two months of treatment, similar proportions of participants in the SoC plus nVNS group (52% (25/48)) and control group (49% (24/49)) reported ≥1 AE; most AEs were mild or moderate (93% (108/116)). Among participants assigned to SoC plus nVNS, 38% (18/48) experienced AEs during the randomised phase and 25% (12/48) experienced AEs in the extension phase. Among individuals assigned to control, 27% (13/49) experienced AEs during the randomised phase and 24% (12/49) experienced AEs in the extension phase. Overall, the most common AEs in any treatment group were CH attacks, headache, nasopharyngitis, dizziness, oropharyngeal pain, and neck pain.
Table 3.
Safety and tolerability (safety population).
| Incidence of AEs | SoC plus nVNS (n = 48) | Control (n = 49) |
|---|---|---|
| Participants with ≥1 AE, n (%) | 25 (52) | 24 (49) |
| Participants with ≥1 AE leading to discontinuation, n (%) | 3 (6)a | 4 (8)b |
| Participants reporting any serious AEc, n (%) | 2 (4) | 2 (4) |
| Participants with ≥1 device-related AE, n (%) | 13 (27)d | 7 (14)e |
| AEs reported in ≥5% of participants in any treatment group, n (%) | ||
| Nervous system disorders | ||
| CH attack | 1 (2)f | 5 (10)f |
| Dizziness | 3 (6)f | 3 (6) |
| Headache | 4 (8) | 1 (2) |
| Infections and infestations | ||
| Nasopharyngitis | 1 (2) | 4 (8) |
| Respiratory, thoracic, and mediastinal disorders | ||
| Oropharyngeal pain | 3 (6)f | 1 (2) |
| Musculoskeletal and connective tissue disorders | ||
| Neck pain | 3 (6) | 0 |
AE: adverse event; CH: cluster headache; nVNS, non-invasive vagus nerve stimulation; SoC: standard of care.
Included feeling hot, malaise, haematoma after scheduled surgery and depressed mood.
Included chest pain, fatigue, depressed mood and CH.
Cholecystitis and haematoma after scheduled surgery were reported in two participants in the SoC plus nVNS group; genital herpes simplex virus infection and exacerbation of CH were reported in two participants in the control group.
Includes depressed mood, malaise, oropharyngeal pain, CH, paraesthesia, muscle twitching, muscle spasms, feeling hot, hot flush, acne, pain, throat tightness, dizziness, hyperhidrosis, toothache, decreased appetite and skin irritation.
Included erythema, facial oedema, CH, chest pain, fatigue, depressed mood, pruritus, musculoskeletal stiffness and parosmia, all of which occurred during the extension phase.
Included ≥1 AE determined by causality assessment to be related to treatment.
Four people (two per treatment group) reported a serious AE (SoC plus nVNS: cholecystitis and haematoma after scheduled surgery; control: genital herpes simplex virus infection and exacerbation of CH; Table 3); no serious AEs were considered related to the nVNS device, and all had resolved by the end of the study. During the randomised phase, 11 individuals who received nVNS experienced 15 device-related AEs, 13 (87%) of which were mild or moderate; severe device-related depression and malaise were noted in one person. Two participants who continued treatment with nVNS during the extension phase experienced mild device-related AEs. Mild or moderate device-related AEs occurred in seven participants who began nVNS therapy during the extension phase. Physical examinations and vital sign assessments at the end of the extension phase revealed no treatment-related abnormalities.
At the end of the study, 65% of participants (62/96) stated that they would recommend the nVNS device to others, >75% of individuals rated nVNS as easy to use and >50% reported some degree of satisfaction with nVNS.
Discussion
Given the limited understanding of the pathophysiology of CH, management strategies have been derived primarily from empirical evidence (10,18,19). Prophylactic therapies for CH have been established on the basis of clinical experience, with very few well-conducted randomised clinical trials providing high-level supportive evidence (4,18). To date, PREVA is the largest prospective, randomised, controlled trial that examined the clinical benefit of prophylactic nVNS therapy in a treatment-refractory chronic CH population. This study met its primary end point by demonstrating that daily adjunctive prophylactic nVNS therapy significantly reduced the number of CH attacks per week, which presumably led to substantial reductions in abortive medication use and meaningful improvements in QoL. Compared with participants who received nVNS treatment during the randomised phase, individuals who began nVNS therapy during the extension phase had a lower response rate. Findings in the mITT population showed that people who persisted in the study continued to respond to nVNS, which is consistent with the reported effects of VNS in epilepsy (20). Treatment with nVNS was safe and well tolerated, with no serious device-related AEs.
Study limitations include the lack of a placebo/sham device, an open-label study design, the short treatment duration and the use of patient-reported outcomes. No placebo arm was incorporated into the study because a suitable placebo/sham device had not yet been designed. In lieu of a placebo/sham arm, SoC was deemed the most appropriate control treatment that was reflective of a real-world clinical scenario. The open-label study design and short treatment duration may have contributed to a placebo effect in both treatment groups. The 16.7% response rate in the control group during the extension phase may partially reflect a placebo response to nVNS. The initial response experienced in the control group during the randomised phase may have also impacted the capacity for a meaningful response to nVNS during the extension phase. Additionally, fewer individuals in the control arm (50%) than in the nVNS arm (64.4%) were highly adherent (≥80%) to prophylactic nVNS, which may have further confounded response rates and reductions in abortive medication use in this group. Only patients with chronic, treatment-refractory CH were included because of their stable CH attack frequency and intensity. A 2.5-month study duration was deemed sufficient to observe a treatment effect. Treatment response in favour of nVNS was consistent across ITT, mITT and per-protocol populations (per-protocol population was defined as participants in the mITT population who had ≥12 days of observation in the randomised phase and no major protocol violation; data not shown). Because no CH-specific QoL instruments exist, the EQ-5D-3L and HIT-6 measures were considered most appropriate, and nVNS prophylaxis resulted in meaningful improvements for both measures. The apparent lack of effect of acute nVNS therapy on CH duration or severity is consistent with findings in the chronic CH population that were reported in a recent study of acute nVNS therapy for CH (21,22). The nVNS adherence rates in this study (50%–64%) are consistent with those reported for prophylactic non-invasive neuromodulation in migraine (23) and are considered meaningful given that twice-daily nVNS requires more effort and participation than a conventional oral medication regimen.
Current prophylactic treatments for CH are limited, with verapamil and lithium being used most often. High-dose verapamil is associated with serious cardiovascular effects (e.g. arrhythmias, bradycardia, oedema) and lethargy (24,25); AEs of lithium therapy include tremor, hypothyroidism and renal dysfunction (19,26). Thus, both therapies may necessitate close patient monitoring, which in turn may incur substantial costs. Long-term occipital nerve stimulation (ONS) has been shown to reduce attack frequency in patients with chronic CH, but its use is hampered by the invasive nature of the procedure and high incidence of AEs (27–30). Deep brain stimulation (DBS) had no effect on attack frequency in patients with chronic CH during the two-month randomised phase of a double-blind crossover study (31); however, >50% reduction in weekly CH attack frequency was noted after 10 months of continuous open-label DBS. In studies of ONS and DBS, implantation-related infections (28,29,31), intracranial haemorrhage (32), electrode/lead displacement (27,29,30), device-related technical problems or other health complications (27,29,31) and lead revisions (30) have been reported. Moreover, the per-patient costs for these therapies are extremely high (27,33). Sphenopalatine ganglion stimulation was found to be effective in the acute treatment of chronic CH; however, procedural complications, infections and other implantation-related AEs were reported (34). Thus, the inherent risks associated with currently available implanted neuromodulation devices and the side effects of prophylactic medications for chronic CH highlight the need for alternative therapies. To this end, nVNS serves as a safe and effective non-invasive therapy that could be easily incorporated into the existing treatment regimens of patients with chronic CH.
We conclude that findings from PREVA demonstrate the clinical relevance of nVNS in patients with treatment-refractory chronic CH. Adjunctive prophylactic therapy with nVNS can safely reduce CH attack frequency and may yield clinical benefits beyond those afforded by SoC treatment.
PREVA Study Group
Investigators are listed by country. 1. Germany: Migraine and Headache Clinic, Königstein – Charly Gaul, MD (principal investigator), Ronald Brand, MD (subinvestigator); University Hospital-Essen, Essen – Hans-Christoph Diener, MD, PhD (principal investigator), Kasja Rabe (subinvestigator), Holle Dagny (subinvestigator), Steffen Nägel, MD (subinvestigator), Maja Bak, MD (subinvestigator); Ludwig Maximilian University of Munich, Munich – Andreas Straube, MD (principal investigator), Bernhard Blum, MD (subinvestigator), Ruth Ruscheweyh, MD (subinvestigator), Ozan Eren, MD (subinvestigator); Department of Neurology, Charité University Hospital, Berlin – Uwe Reuter, MD (principal investigator), Heike Israel-Willner, MD (subinvestigator), Lars Neeb, MD (subinvestigator); Krankenhaus Lindenbrunn, Lindenbrunn – Stefan Evers, MD, PhD (principal investigator); 2. United Kingdom: The Walton Centre for Neurology and Neurosurgery, Liverpool – Nicholas Silver, MBBS, PhD (principal investigator), Helen Banks, MD (subinvestigator), Heike Arndt, MD (subinvestigator); The Southern General Hospital, Glasgow – Alok Tyagi, MD (principal investigator); Hull Royal Infirmary, Hull – Fayyaz Ahmed, MD (principal investigator), Anwar Osman, MD (subinvestigator); 3. Belgium: Liège University, Liège – Delphine Magis, MD, PhD (principal investigator), Jean Schoenen, MD (subinvestigator); 4. Italy: Sant’Andrea Hospital, Sapienza University of Rome, Rome – Paolo Martelleti, MD (principal investigator), Andrea Negro, MD (subinvestigator).
Article highlights
Adjunctive prophylactic non-invasive vagus nerve stimulation (nVNS) therapy reduced the number of cluster headache (CH) attacks per week and use of abortive medication and improved quality of life (QoL) in individuals with chronic CH.
Treatment with nVNS was safe and well tolerated.
In patients with chronic CH, adjunctive prophylactic nVNS may provide clinical benefits beyond those derived from their current standard of care (SoC).
Acknowledgements
The authors would like to acknowledge all of the investigators and study sites. The PREVA study investigators acknowledge the contributions of all the nurses and study coordinators. Medical writing support for this manuscript was provided by Regina Switzer, PhD, Kalpana Vijayan, PhD, and Michael Zbreski, PharmD, of MedLogix Communications, LLC (Schaumburg, IL, USA).
Dr Charly Gaul, Dr Hans-Christoph Diener, Eric J. Liebler and Annelie Andersson contributed to the PREVA study design. All primary investigators (i.e. Drs Charly Gaul, Hans-Christoph Diener, Uwe Reuter, Nicholas Silver, Delphine Magis and Andreas Straube) were involved in participant recruitment and treatment for the PREVA study. All authors participated in data collection, interpretation and validation. Annelie Andersson, Eric J. Liebler and NAMSA were involved in data analysis. All authors reviewed, critiqued and contributed to revision of the manuscript content and provided approval of the final manuscript draft to be submitted to Cephalalgia. The corresponding author, Dr Charly Gaul, had full access to all the study data and had final responsibility for the decision to submit the manuscript for publication. None of the authors received compensation from a pharmaceutical company or other agency for their contributions to the development of this manuscript.
Funding
This work was supported by electroCore, LLC, which included professional writing and editorial support from MedLogix Communications and data analysis support from North American Science Associates (NAMSA).
Declaration of conflicting interests
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article. Dr Charly Gaul has received honoraria from Allergan, Berlin-Chemie, MSD, electroCore, St. Jude Medical, Grünenthal, Desitin, Bayer, Boehringer Ingelheim, Autonomic Technologies and Hormosan. Dr Gaul has no ownership interests and does not own any pharmaceutical company stocks. Dr Andreas Straube has received honoraria from Allergan, Berlin-Chemie, Desitin, MSD, Pfizer, electroCore, Boehringer Ingelheim and St. Jude Medical. He has also received grants from the German Science Council, the German Secretary of Education, the Kröner-Fresenius Foundation and the University of Munich. Dr Hans-Christoph Diener has received honoraria for participation in clinical trials and for contributions to advisory boards and oral presentations sponsored by Addex Pharma, Adler, Allergan, Almirall, Amgen, Autonomic Technologies, AstraZeneca, Bayer, Vital, Berlin-Chemie, Boehringer Ingelheim, Bristol-Myers Squibb, Chordate Medical, Coherex Medical, CoLucid Pharmaceuticals, electroCore, GlaxoSmithKline, Grünenthal, Janssen-Cilag, Labrys Biologics, Eli Lilly, La Roche, 3 M Medica, Medtronic, Menarini, Minster Pharmaceuticals, MSD, NeuroScore, Novartis, Johnson & Johnson, Pierre Fabre, Pfizer, Schaper and Brümmer, Sanofi, St. Jude Medical and Weber & Weber. Dr Diener has also received research funding from Allergan, Almirall, AstraZeneca, Bayer, electroCore, GlaxoSmithKline, Janssen-Cilag, MSD and Pfizer; he has received additional research support from the German Research Council, the German Ministry of Education and Research and the European Union. Dr Diener has no ownership interests and does not own any pharmaceutical company stocks. Dr Uwe Reuter has received honoraria for participation in advisory boards, lectures and clinical trials sponsored by Allergan, Amgen, Autonomic Technologies, CoLucid Pharmaceuticals, Johnson & Johnson, Eli Lilly, electroCore, Haas & Health Partner, Hormosan, MSD and Merck Sharp & Dohme. Dr Nicholas Silver has received honoraria from Allergan and electroCore and investigator fees paid to the Walton Centre. Dr Delphine Magis has received travel grants from Allergan, Bayer, Biogen Idec and electroCore and advisory board fees from Medtronic. Annelie Andersson and Eric J. Liebler are both employees of electroCore and receive stock ownership.
References
- 1.Fischera M, Marziniak M, Gralow I, et al. The incidence and prevalence of cluster headache: A meta-analysis of population-based studies. Cephalalgia 2008; 28: 614–618. [DOI] [PubMed] [Google Scholar]
- 2.Nesbitt AD, Goadsby PJ. Cluster headache. BMJ 2012; 344: e2407–e2407. [DOI] [PubMed] [Google Scholar]
- 3.Headache Classification Committee of the International Headache Society. The International Classification of Headache Disorders. 3rd ed. (beta version). Cephalalgia 2013; 33: 629–808. [DOI] [PubMed] [Google Scholar]
- 4.Francis GJ, Becker WJ, Pringsheim TM. Acute and preventive pharmacologic treatment of cluster headache. Neurology 2010; 75: 463–473. [DOI] [PubMed] [Google Scholar]
- 5.Cohen AS, Burns B, Goadsby PJ. High-flow oxygen for treatment of cluster headache: A randomized trial. JAMA 2009; 302: 2451–2457. [DOI] [PubMed] [Google Scholar]
- 6.Law S, Derry S, Moore RA. Triptans for acute cluster headache. Cochrane Database Syst Rev 2013; 7: CD008042–CD008042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ashkenazi A, Schwedt T. Cluster headache – acute and prophylactic therapy. Headache 2011; 51: 272–286. [DOI] [PubMed] [Google Scholar]
- 8.Leroux E, Valade D, Taifas I, et al. Suboccipital steroid injections for transitional treatment of patients with more than two cluster headache attacks per day: A randomised, double-blind, placebo-controlled trial. Lancet Neurol 2011; 10: 891–897. [DOI] [PubMed] [Google Scholar]
- 9.Nesbitt AD, Marin JC, Tompkins E, et al. Initial use of a novel noninvasive vagus nerve stimulator for cluster headache treatment. Neurology 2015; 84: 1249–1253. [DOI] [PubMed] [Google Scholar]
- 10.Goadsby PJ. Pathophysiology of cluster headache: A trigeminal autonomic cephalgia. Lancet Neurol 2002; 1: 251–257. [DOI] [PubMed] [Google Scholar]
- 11.Bossut DF, Maixner W. Effects of cardiac vagal afferent electrostimulation on the responses of trigeminal and trigeminothalamic neurons to noxious orofacial stimulation. Pain 1996; 65: 101–109. [DOI] [PubMed] [Google Scholar]
- 12.Beekwilder JP, Beems T. Overview of the clinical applications of vagus nerve stimulation. J Clin Neurophysiol 2010; 27: 130–138. [DOI] [PubMed] [Google Scholar]
- 13.Oshinsky ML, Murphy AL, Hekierski H, Jr, et al. Noninvasive vagus nerve stimulation as treatment for trigeminal allodynia. Pain 2014; 155: 1037–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.EQ-5D-3L User guide version 5.1, http://www.euroqol.org/fileadmin/user_upload/Documenten/PDF/Folders_Flyers/EQ-5D-3L_UserGuide_2015.pdf (2015, accessed 12 June 2015).
- 15.HIT-6™ Headache Impact Test, http://neurohealth.info/wp-content/uploads/2010/10/hit6.pdf (2001, accessed 28 August 2014).
- 16.Walters SJ, Brazier JE. Comparison of the minimally important difference for two health state utility measures: EQ-5D and SF-6D. Qual Life Res 2005; 14: 1523–1532. [DOI] [PubMed] [Google Scholar]
- 17.Coeytaux RR, Kaufman JS, Chao R, et al. Four methods of estimating the minimal important difference score were compared to establish a clinically significant change in Headache Impact Test. J Clin Epidemiol 2006; 59: 374–380. [DOI] [PubMed] [Google Scholar]
- 18.Leone M, Franzini A, Cecchini AP, et al. Cluster headache: Pharmacological treatment and neurostimulation. Nat Clin Pract Neurol 2009; 5: 153–162. [DOI] [PubMed] [Google Scholar]
- 19.May A. Cluster headache: Pathogenesis, diagnosis, and management. Lancet 2005; 366: 843–855. [DOI] [PubMed] [Google Scholar]
- 20.Murphy JV. and The Pediatric VNS Study Group. Left vagal nerve stimulation in children with medically refractory epilepsy. J Pediatr 1999; 134: 563–566. [DOI] [PubMed] [Google Scholar]
- 21.Silberstein SD, Mechtler L, Kudrow DB, et al. Efficacy and safety outcomes of non-invasive vagus nerve stimulation for the acute treatment (ACT1) of cluster headache study. In: 57th annual meeting of the American Headache Society, Washington, DC, USA, 18–21 June 2015.
- 22.Tepper SJ, Silberstein SD, Mechtler L, et al. Predefined exploratory outcomes from the study of non-invasive vagus nerve stimulation from the Acute Treatment (ACT1) of Cluster Headache. In: 57th annual meeting of the American Headache Society, Washington, DC, USA, 18–21 June 2015.
- 23.Schoenen J, Vandersmissen B, Jeangette S, et al. Migraine prevention with a supraorbital transcutaneous stimulator: A randomized controlled trial. Neurology 2013; 80: 697–704. [DOI] [PubMed] [Google Scholar]
- 24.Lantéri-Minet M, Silhol F, Piano V, et al. Cardiac safety in cluster headache patients using the very high dose of verapamil (≥720 mg/day). J Headache Pain 2011; 12: 173–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cohen AS, Matharu MS, Goadsby PJ. Electrocardiographic abnormalities in patients with cluster headache on verapamil therapy. Neurology 2007; 69: 668–675. [DOI] [PubMed] [Google Scholar]
- 26.Baek JH, Kinrys G, Nierenberg AA. Lithium tremor revisited: Pathophysiology and treatment. Acta Psychiatr Scand 2014; 129: 17–23. [DOI] [PubMed] [Google Scholar]
- 27.Mueller O, Diener HC, Dammann P, et al. Occipital nerve stimulation for intractable chronic cluster headache or migraine: A critical analysis of direct treatment costs and complications. Cephalalgia 2013; 33: 1283–1291. [DOI] [PubMed] [Google Scholar]
- 28.Fontaine D, Christophe Sol J, Raoul S, et al. Treatment of refractory chronic cluster headache by chronic occipital nerve stimulation. Cephalalgia 2011; 31: 1101–1105. [DOI] [PubMed] [Google Scholar]
- 29.Magis D, Gerardy PY, Remacle JM, et al. Sustained effectiveness of occipital nerve stimulation in drug-resistant chronic cluster headache. Headache 2011; 51: 1191–1201. [DOI] [PubMed] [Google Scholar]
- 30.Brewer AC, Trentman TL, Ivancic MG, et al. Long-term outcome in occipital nerve stimulation patients with medically intractable primary headache disorders. Neuromodulation 2013; 16: 557–562. [DOI] [PubMed] [Google Scholar]
- 31.Fontaine D, Lazorthes Y, Mertens P, et al. Safety and efficacy of deep brain stimulation in refractory cluster headache: A randomized placebo-controlled double-blind trial followed by a 1-year open extension. J Headache Pain 2010; 11: 23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schoenen J, Di Clemente L, Vandenheede M, et al. Hypothalamic stimulation in chronic cluster headache: A pilot study of efficacy and mode of action. Brain 2005; 128: 940–947. [DOI] [PubMed] [Google Scholar]
- 33.Jenkins B, Tepper SJ. Neurostimulation for primary headache disorders: Part 2, review of central neurostimulators for primary headache, overall therapeutic efficacy, safety, cost, patient selection, and future research in headache neuromodulation. Headache 2011; 51: 1408–1418. [DOI] [PubMed] [Google Scholar]
- 34.Schoenen J, Jensen RH, Lantéri-Minet M, et al. Stimulation of the sphenopalatine ganglion (SPG) for cluster headache treatment. Pathway CH-1: A randomized, sham-controlled study. Cephalalgia 2013; 33: 816–830. [DOI] [PMC free article] [PubMed] [Google Scholar]