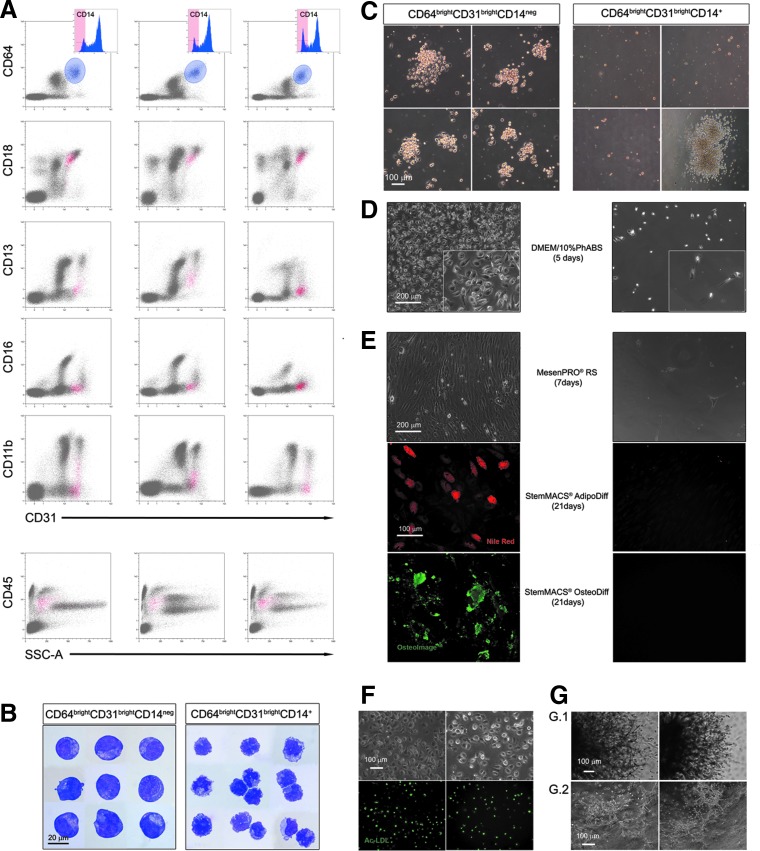
FIG. 4.
Pop#8 isolation by their unique CD64brightCD31brightCD14neg phenotype. Gating events for bright expression of both CD31 and CD64 (light blue gate) most cells showed to be CD14+. CD64brightCD31brightCD14neg minor population plotted exclusively in the R8 region of CD18 versus CD31 cytogram (red dots), confirming that this population was the Pop#8 population (three representative samples are presented in (A). CD45 was mildly expressed as well as CD13, CD16, and CD11b. CD64brightCD31brightCD14neg appeared to be large cells with blast-like morphology, in sharp contrast with the monocytic morphology showed by the CD14+ counterpart (B). Applying hematopoietic colony-forming assay, CD64brightCD31brightCD14neg generated clusters of large cells above few adherent MPC-like cells not detected culturing the CD14+ counterpart, where GM- or M-CFUs were occasionally reported (C). Consistently with LinnegCD31brightCD18dim/int, CD64brightCD31brightCD14neg cells generated MPCs (left panel) (D) when cultured in DMEM/10%PhABS. Those MPCs rapidly differentiated into MSCs, which retained their adipogenic and osteogenic potential (left panels) (E). The CD14+ counterpart failed to generate adherent cultures (right panels) (D, E). Pop#8-derived MPCs were also capable of Ac-LDL uptake (green in F) and angiogenic sprouting (G.1) that primed the cells to form capillary tube-like structures (G.2). Ac-LDL, acetylated low-density lipoprotein; GM-CFUs, granulocyte/macrophage colony-forming units; M-CFUs, macrophage colony-forming units.