Figure 4.
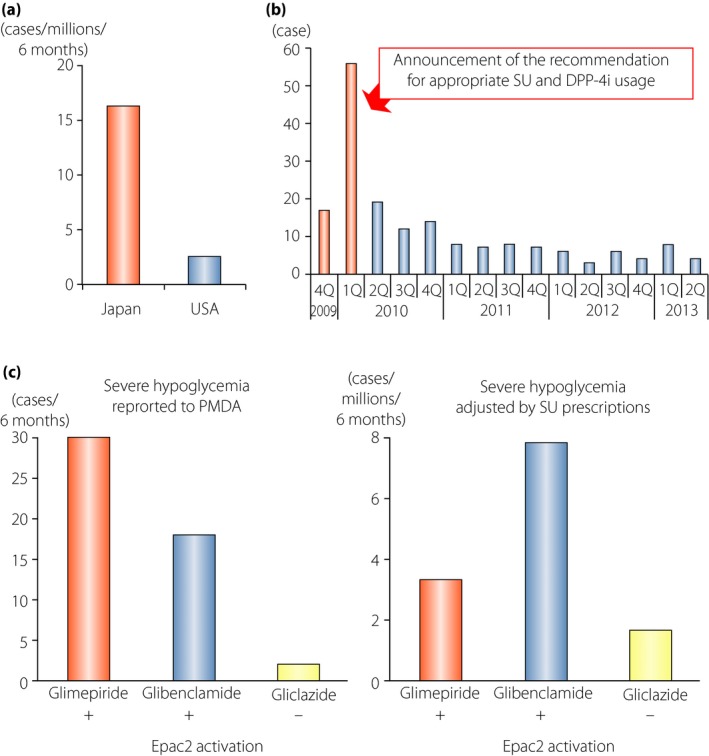
Severe hypoglycemia in individuals receiving dipeptidyl peptidase‐4 inhibitors (DPP‐4i) as add‐on to sulfonylureas (SUs). (a) Comparison of the incidence rate of severe hypoglycemia in individuals receiving the DPP‐4i, sitagliptin, in Japan and the USA. The incidence of hypoglycemic coma with sitagliptin was 16.3 per million patients who received sitagliptin during the first 6 months after its launch in Japan, and was approximately 6.4‐fold higher than that of USA in the corresponding period. (b) Transition in cases of severe hypoglycemia in individuals treated with sitagliptin in each quarter. The number was drastically reduced on announcement of the recommendation from the committee for appropriate use of incretin‐related drugs (glucagon‐like peptide‐1 receptor agonists and DPP‐4 inhibitors). (c) Comparison of the numbers of severe hypoglycemia cases in individuals receiving sitagliptin as add‐on to indicated SUs. Left, the numbers of cases reported to the Japanese Pharmaceuticals and Medical Devices Agency. Right, estimated incidence rates calculated by dividing the numbers of cases reported to the Japanese Pharmaceuticals and Medical Devices Agency by the numbers of individual prescriptions of indicated SUs combination with sitagliptin in the same period. EPAC2, exchange protein directly activated by cyclic adenosine monophosphate 2. Reproduced from Yabe and Seino39 with permission.
