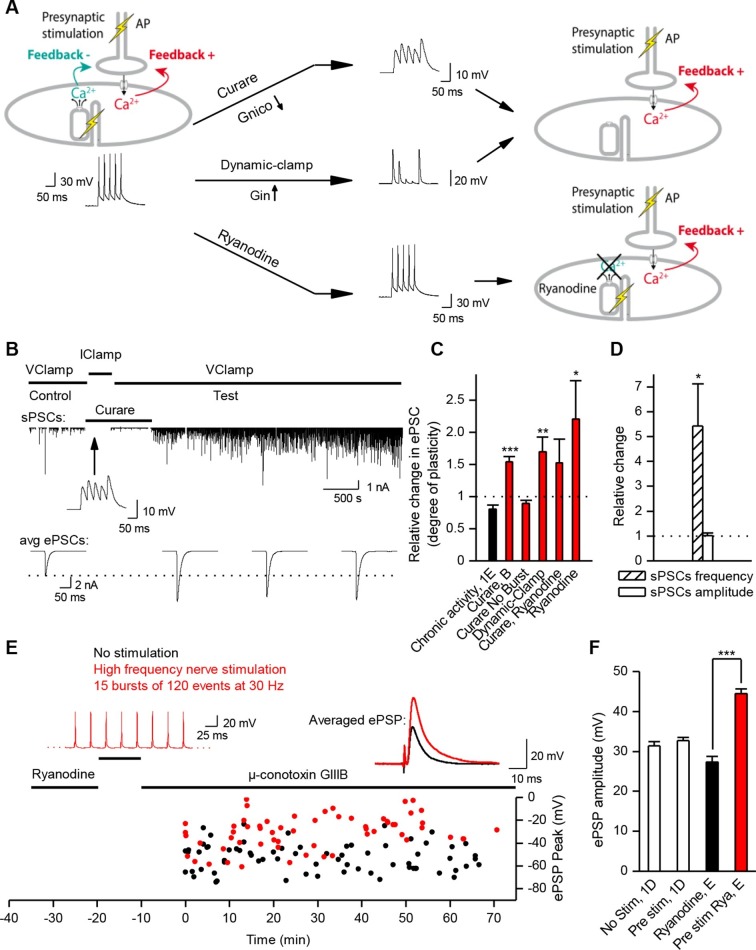
Figure 3. Nicotinic receptor activity induces a positive feedback on ACh release.
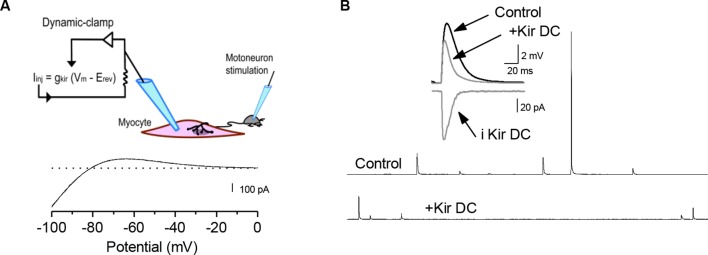
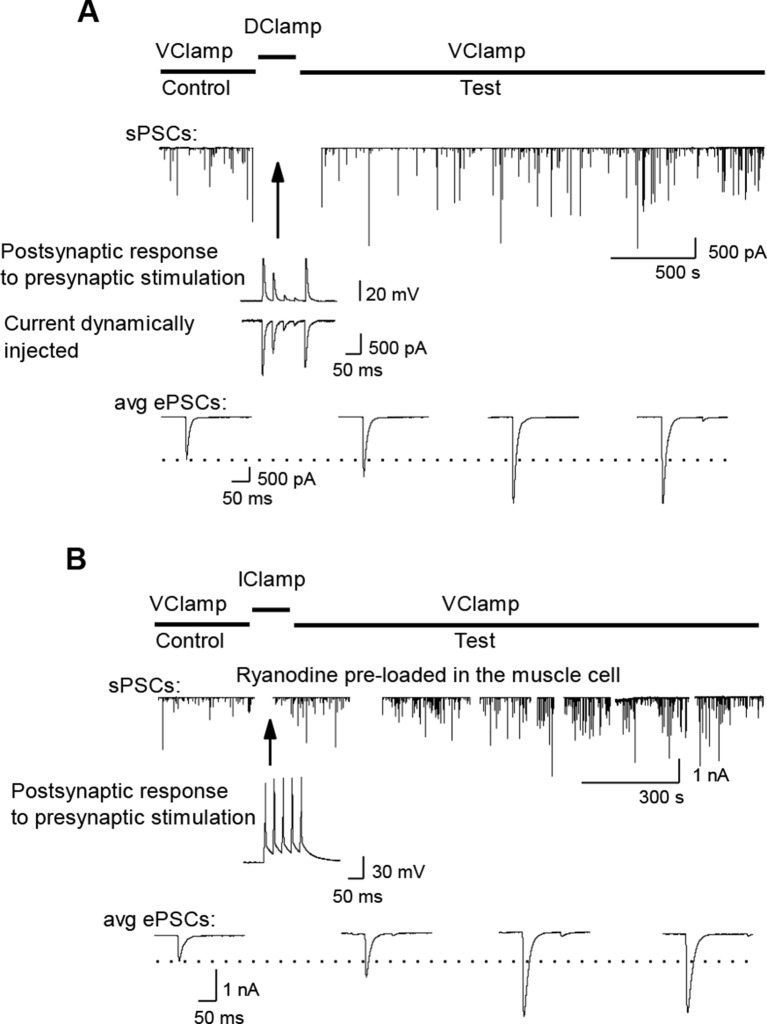
(A) In order to obtain the nicotinic calcium signal in absence of DICR in Xenopus, synaptic events were transiently kept subthreshold either with curare (decreased nicotinic conductance, Gnico) or by dynamic-clamp injection of gK+ leaks (increased input conductance, Gin), or left suprathreshold while DICR was blocked by ryanodine. (B) Effect of presynaptic burst stimulation and curare on spontaneous (upper trace, sPSC) and evoked synaptic currents (ePSC). ePSCs recorded under voltage-clamp were evoked at low rate (0.03 Hz) and averaged by 30–40 events (lower traces). During conditioning presynaptic stimulations (3 bursts of 5 events at 30 Hz), the postsynaptic potential was released from clamp and curare transiently applied (middle trace). Upper trace: for clarity, ePSCs were removed from the continuous trace in order to display the sPSCs only. (C), In Xenopus, mean ePSC relative change 30 min after control chronic bursting activity ('chronic activity', n = 5, illustrated in Figure 1E), 45 min after subthreshold synaptic activity ('Curare', n = 9, illustrated in B; 'Dynamic-clamp', n = 5, illustrated in Figure 3—figure supplement 2A), transient curare application in absence of stimulation ('curare No Burst', n = 3), sub- ('curare-ryanodine', n = 3) and suprathreshold synaptic activity ('ryanodine', n = 6, illustrated in Figure 3— figure supplement 3) in muscle cells preloaded with ryanodine. (D), Relative change in amplitude and frequency of sPSCs after potentiation in Xenopus. (E), In FDB mice muscles, voltage reached by ePSPs in ryanodine treated (black dots, n = 60 fibers, 2 mice), and in ryanodine treated and burst stimulated preparations (red dots, n = 70 fibers, 2 mice). (F) Mean ePSP amplitude in control ('no stim') and high frequency nerve stimulation ('Pre stim') shown in Figure 1D, in non-stimulated ('Ryanodine') and in high frequency stimulated ('Pre stim Rya') ryanodine treated preparations shown in E. *, p<0.05; **, p<0.01; ***, p<0.001; t-test.