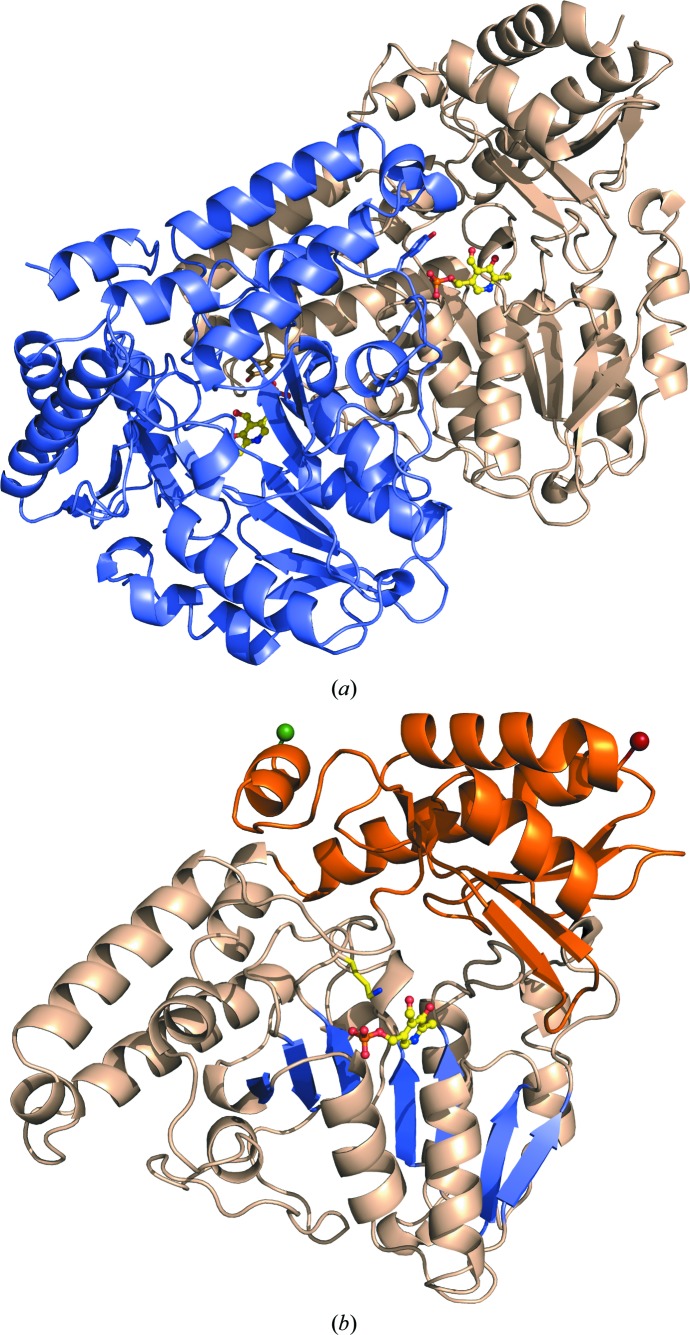
Figure 2.
Three-dimensional structure of PvdN. (a) The secondary structure is depicted for both subunits in the homodimer, with one subunit in wheat and the other in light blue. The PLP cofactor is shown in ball-and-stick representation with yellow C atoms, blue N atoms, red O atoms and orange P atoms. The tyrosine caps (Tyr308) contributing to the ligand pocket from each adjacent monomer are highlighted as sticks. (b) A single subunit of the homodimer with the N-terminal residue as a green sphere and the C-terminal residue as a red sphere. The small domain is shown in orange and the large domain in wheat. The seven-stranded β-sheet (β2–β8) in the large domain is highlighted in blue, with the PLP cofactor shown in ball-and-stick representation (yellow C atoms, blue N atoms, red O atoms and orange P atoms). The active-site lysine (Lys264) off the loop connecting β7 and β8 is shown extended towards the PLP.