Intracellular colonization of arbuscular mycorrhizal fungi is rapidly and temporarily inhibited by P and is stabilized by plant symbiotic P transporter.
Abstract
Phosphorus (P) is a crucial nutrient for plant growth, but its availability to roots is limited in soil. Arbuscular mycorrhizal (AM) symbiosis is a promising strategy for improving plant P acquisition. However, P fertilizer reduces fungal colonization (P inhibition) and compromises mycorrhizal P uptake, warranting studies on the mechanistic basis of P inhibition. In this study, early morphological changes in P inhibition were identified in rice (Oryza sativa) using fungal cell wall staining and live-cell imaging of plant membranes that were associated with arbuscule life cycles. Arbuscule density decreased, and aberrant hyphal branching was observed in roots at 5 h after P treatment. Although new arbuscule development was severely inhibited, preformed arbuscules remained intact and longevity remained constant. P inhibition was accelerated in the rice pt11-1 mutant, which lacks P uptake from arbuscule branches, suggesting that mature arbuscules are stabilized by the symbiotic P transporter under high P condition. Moreover, P treatment led to increases in the number of vesicles, in which lipid droplets accumulated and then decreased within a few days. The development of new arbuscules resumed within by 2 d. Our data established that P strongly and temporarily inhibits new arbuscule development, but not intraradical accommodation of AM fungi.
Phosphorus (P) is a crucial major nutrient for plant growth but is also one of the most easily depleted nutrients around roots because of its low mobility in soil (Bieleski, 1973; Vance, 2001). As a consequence, plants have evolved various strategies to acquire soil P, and arbuscular mycorrhizal (AM) symbiosis forms an integral part of P acquisition systems in land plants. Plants supply AM fungi with organic compounds to build mycelium, which ramifies through the soil up to several centimeters from the root surface (Smith et al., 2011). P is then translocated through syncytial mycelia to the roots and is released to interfacial apoplasts from arbuscules, which are the highly branched structures of AM fungi in cortical cells. Increased spatial exploitation by hyphae in soil (Marschner, 1995) and greater P acquisition ability of mycorrhizal roots enable the host plant to improve nutrient uptake and in many cases leads to increased biomass accumulation in comparison with nonmycorrhizal condition.
Calculations of the contribution of P uptake via AM fungi (mycorrhizal pathway) to total plant P uptake suggest that mycorrhizal pathways dominate total P uptake under low P conditions (Smith et al., 2004; Yang et al., 2012). Accordingly, mycorrhizal symbiosis down-regulates plant P transporter genes that may contribute to the direct pathway involving P uptake at the root epidermis or root hairs (Javot et al., 2007; Walder et al., 2015). Low P uptake through direct pathways and increased P contents in mycorrhizal plants reflect superior P uptake via the mycorrhizal pathway. However, the application of inorganic P fertilizer has been shown to significantly reduce AM development in a number of studies (Baylis, 1967; Mosse, 1973; Sanders and Tinker, 1973; Breuillin et al., 2010; Balzergue et al., 2011) and is referred to as P inhibition (Graham et al., 1981). Given that mycorrhizal roots have higher P uptake under low P conditions, the formation of mycorrhizal symbiosis in the presence of high P conditions may also increase P uptake. Although the mechanisms behind P inhibition have been investigated in numerous morphological, physiological, and molecular studies, the primary causes of P inhibition remain unknown (Balzergue et al., 2013).
Early morphological studies showed that P inhibition at the very early stage entirely reflects the reduced growth of “infection units” (Braunberger et al., 1991; Bruce et al., 1994) that comprise internal mycelium arising from entry points (Cox and Sanders, 1974; Walker and Smith, 1984). Fungal colonization processes that contribute to the growth of infection units include (1) preinfection growth of hyphae through the soil or the growth of runner hyphae from adjoining infection units, (2) formation of entry points (hyphopodia) on the root surface, (3) longitudinal extension of intercellular or intracellular hyphae within roots of Arum-type or Paris-type mycorrhiza, respectively, and (4) formation of arbuscules in cortical cells (Cox and Sanders, 1974; Bonfante-Fasolo, 1984). Arbuscule maturation takes less than 1 d to occupy most of the cell space, and the arbuscules are functional for only 2 to 3 d (Kobae and Hata, 2010; Kobae and Fujiwara, 2014). Bruce et al. (1994) showed that neither the duration of the preinfection phase nor the rate of new entry point formation is affected by P concentration. In agreement, Medicago truncatula roots maintained their cell responses to perceive fungal partners even under high P conditions, as indicated by nuclear calcium spiking of subhyphopodia root epidermal cells, which is a hallmark of AM symbiotic signaling (Singh and Parniske, 2012; Balzergue et al., 2013). Balzergue et al. (2011) showed that the number of hyphopodia is significantly reduced upon 4 to 7 weeks of high P treatment; however, a long-term P treatment can reduce the formation of secondary entry points through reduced growth of external mycelium from an established infection (Schwab et al., 1983; Braunberger et al., 1991). Therefore, P inhibition may primarily occur inside roots, although the precise stages of P inhibition during the growth of infection units remains poorly understood.
To investigate the stages of P inhibition, we performed detailed morphological analyses of intraradical development during short periods after P treatment. In these experiments, rice (Oryza sativa) seedlings expressing the arbuscule life cycle marker GFP-AM42 (Kobae and Fujiwara, 2014) and the symbiotic phosphate transporter PT11-GFP (Kobae and Hata, 2010) were used with a live imaging system (Kobae and Fujiwara, 2014). Subsequent observations revealed both fragile and robust colonization under conditions of P inhibition. Specifically, P selectively inhibited the development of new arbuscules without compromising the life cycle of finely branched arbuscules or eliminating the intraradical accommodation of AM fungi. Importantly, the reduction of arbuscule density with P was accelerated in the rice pt11-1 mutant, which is defective in P uptake after release from arbuscule branches (Yang et al., 2012). These observations suggest a novel regulatory mode of intraradical colonization that is mycelium-type specific during P inhibition.
RESULTS
P Rapidly Reduces Arbuscule Density in Hyphal-Colonized Regions
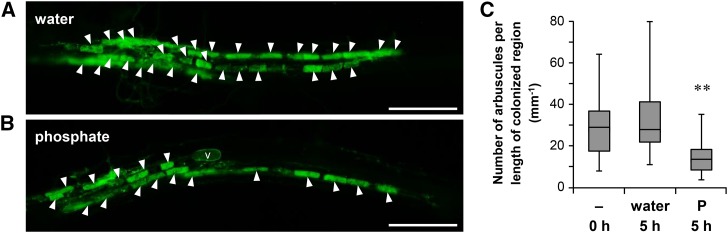
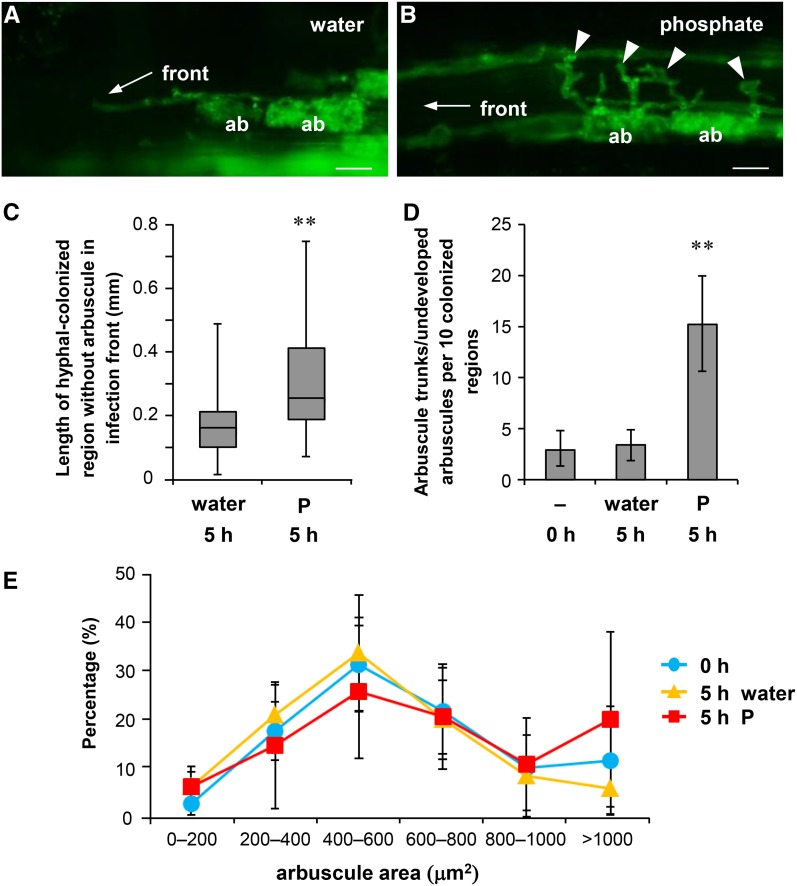
To investigate the effects of P treatment on colonization of AM fungi, we initially inoculated rice seedlings with Rhizophagus irregularis and examined the morphological changes shortly after exposure to high P conditions. At 14 d post-planting (dpp), plants were treated with 20 mL of a 0.5 mM P solution. At this stage, the roots were well colonized with AM fungi, and the morphological changes of intraradical mycelium were analyzed using wheat germ agglutinin-conjugated fluorescein isothiocyanate (WGA-FITC), which selectively stains fungal cell walls (Bonfante-Fasolo et al., 1990). The number of arbuscules in the colonized region tended to decrease at 5 h after P treatment (Fig. 1, A and B). The term “colonized region” was used in reference to hyphal-colonized root regions and single colonized regions were bound by two infection fronts comprising intercellular hyphae (Supplemental Fig. S1; Buwalda et al., 1984). Numerous infection units can coalesce to form a colonized region, where the border of each infection unit is not identified at least within a few days from the beginning of colonization (Sanders and Sheikh, 1983). The lengths of colonized regions and numbers of arbuscules with fine branches were determined for each. Arbuscule densities in the colonized regions significantly decreased at 5 h after P treatment compared with those after water treatment (Fig. 1C). In the infection fronts of water-treated roots, the growing tips of intercellular hyphae tended to be followed immediately by arbuscule formations (Fig. 2A). However, in the infection fronts of P-treated roots, the lengths of hyphal-colonized regions without finely branched arbuscules increased (Fig. 2C; Supplemental Fig. S1B). In contrast, arbuscule trunks/undeveloped arbuscules characterized by coarse and lower order branching compared with mature arbuscules were formed at regular intervals on intercellular hyphae (Fig. 2B; Supplemental Fig. S2). The numbers of arbuscule trunks/undeveloped arbuscules in the infection fronts of P-treated roots were significantly higher than those in the control roots (Fig. 2D). Despite decreases in arbuscule density in the infection fronts of P-treated roots, the lengths of colonized regions and densities of hyphopodia over the lengths of colonized regions were not affected by P treatment at 20 h (Supplemental Fig. S3). Moreover, the size of finely branched arbuscules in the colonized regions was not affected by P treatment (Fig. 2E).
Figure 1.
P treatment rapidly reduces arbuscule density in colonized regions. The roots of rice colonized with R. irregularis were treated with water (A) or 0.5 mM Pi (B) at 14 dpp for 5 h in pot culture. The roots were stained with WGA-FITC to detect the intraradical mycelium. Bars = 200 µm. C, The numbers of arbuscules per length of colonized region (arbuscule density). Colonized regions (n = 20–26) were randomly chosen and dissected from three plants, and the longitudinal lengths and numbers of arbuscules with fine branches in each colonized region were measured. Young arbuscules with only trunk or senescent arbuscules that were sparsely stained with WGA-FITC were not included. Middle lines of box plots represent median values, and bars represent ranges (minimum to maximum). **P < 0.01, Welch’s t test (water versus P at 5 h). Arrowheads indicate finely branched arbuscules. V, Vesicle.
Figure 2.
Aberrant hyphal branches were observed at infection fronts of P-treated roots. Rice roots colonized with R. irregularis (14 dpp) were treated with water or 0.5 mM P for 5 h in pot culture and stained with WGA-FITC. A, Image of infection fronts of hyphal-colonized regions treated with water. B, Aberrant hyphal branches were observed in P-treated rice roots (arrowheads). Bars = 20 µm in A and B. C, The lengths of hyphal-colonized regions without finely branched arbuscules at infection fronts. The lengths of intercellular hyphae from the tip of growing hyphal fronts to the outermost branched arbuscule were measured. Data were obtained from 43 (water treatment) or 45 (P treatment) colonized regions that were randomly chosen from three plants. Middle lines of box plots represent median values, and bars represent ranges (minimum to maximum). D, The numbers of arbuscule trunks/undeveloped arbuscules in 10 infection fronts. Ten infection fronts were randomly chosen from three plants of each treatment, and the numbers of arbuscule trunks/undeveloped arbuscules were counted over 200 µm from the tip of the infection front. **P < 0.01, Welch’s t test (P versus water). E, The size distribution of branched arbuscules. Fifteen colonized regions were randomly chosen from three plants, and the areas of longitudinal optical sections of all visible branched arbuscules (29–84 arbuscules from each colonized region) were measured. Data are presented as means ± sd (D and E). ab, Arbuscule.
P Treatment Temporarily Inhibits New Arbuscule Development at Infection Fronts
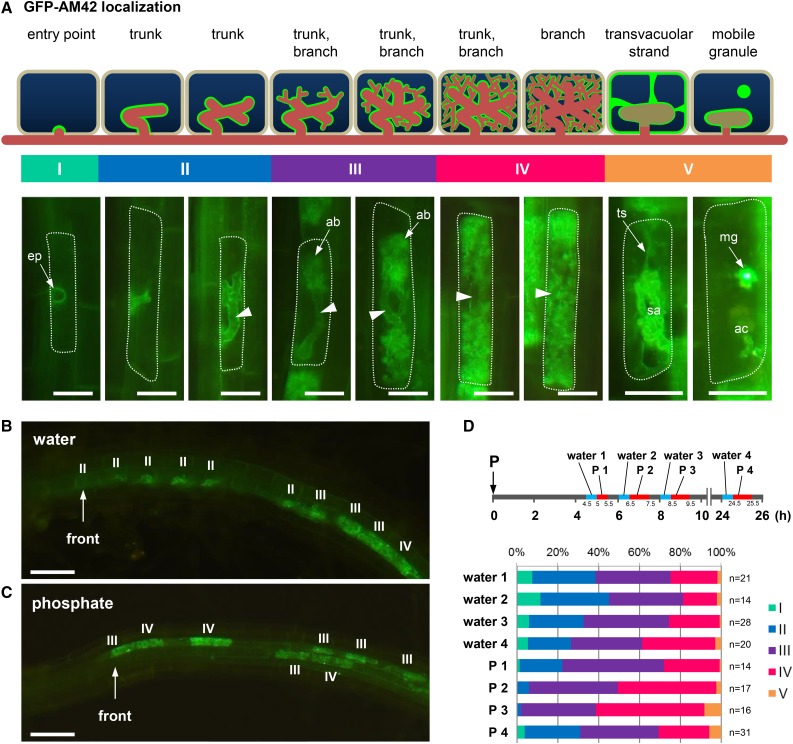
Differential effects of P inhibition at different developmental stages of arbuscules were investigated. In these experiments, young, developing, mature, and collapsed arbuscules were distinguished according to intracellular localization patterns of GFP-AM42/secretory carrier membrane protein (Kobae and Fujiwara, 2014). Accordingly, the effects of P inhibition were determined by evaluating localization patterns of GFP-AM42 throughout the arbuscule life cycle. In these experiments, (1) GFP-AM42 accumulated at fungal entry points of infected cells; (2) outlines of trunks in developing arbuscules were clearly stained with GFP-AM42, and those of fully branched arbuscules were hardly stained; and (3) transvacuolar strands were colocalized with GFP-AM42 in cells containing collapsing arbuscules (Fig. 3A). Taken with data from a previous study (Kobae and Fujiwara, 2014), these observations indicated that the life cycle of arbuscules can be precisely examined in the following five stages (Fig. 3A): Stage I, preinfection or penetration; Stage II, only arbuscule trunk with no visible branches; Stage III, young arbuscule with branches occupying < 70% of the cell area; Stage IV, mature arbuscule with branches occupying >70% of cell area; and Stage V, collapsed arbuscules (Gutjahr and Parniske, 2013). According to this criterion, we investigated the changes in arbuscule life cycles after P treatment concerning the developmental stages of the youngest 10 intracellular colonizations in each infection front. Gradual development of arbuscules can be observed in this region (Buwalda et al., 1984) allowing the precise observation of P inhibition during colonization stages. The numbers of young arbuscules in infection fronts of P-treated roots tended to be decreased compared to those of water-treated roots. Approximately 70% of colonization was in stages I, II, and III at 8 to 8.5 h after the water treatment of rice roots, and approximately 30% was in stages I and II (Fig. 3, B and D). In contrast, a small number of arbuscules in young stages was observed in the infection fronts of P-treated roots (Fig. 3C), with ∼40% of arbuscules being in stages I, II, and III at 8.5 to 9.5 h after P treatment and 2% being in stages I and II (Fig. 3D). The arbuscules of stage I and II recovered at 24.5 to 25.5 h after P treatment. These observations suggest that P treatment rapidly and temporarily inhibited the formation of new arbuscules but has little impact on the formation of mature arbuscules. In agreement, no changes in localization patterns of GFP-AM42 were observed in cells with branched arbuscules (Supplemental Fig. S4).
Figure 3.
P treatment temporarily inhibits new arbuscule development at infection fronts. A, Diagram of arbuscule life cycle based on the intracellular localization patterns of the GFP-conjugated arbuscule life cycle marker (GFP-AM42) in arbusculated cells. proAM42-GFP-AM42 rice plants were inoculated with R. irregularis (14–16 dpp). Representative images of GFP-AM42 in host cells at respective arbuscule developmental stages (bottom panel) are shown as schematic diagrams (upper panel). Stage I, primary entry points are marked by the accumulation of GFP-AM42; Stage II, outlines of young arbuscules, mostly with trunks, were visualized using GFP-AM42; Stage III, developing arbuscules with thick or fine branches; Stage IV, mature arbuscules were rich in fine arbuscule branches (ab) that occupy most of the cell space; Stage V, collapsed arbuscule. Transvacuolar strands (ts) were observed in cells containing senescent arbuscules (sa). Cells with arbuscule clumps (ac) often contained mobile granular structures (mg) that were strongly stained with GFP-AM42 (Kobae and Fujiwara, 2014). In the diagram, green indicates the locations of GFP-AM42 protein, red indicates hyphae of AM fungi, and arrowheads indicate positions of trunks. Representative image of infection fronts of proAM42-GFP-AM42 roots at 5 h after treatment with water (B) or 0.5 mM P (C). D, Ratios of developmental stages of the youngest 10 arbuscules at infection fronts. proAM42-GFP-AM42 roots were treated with water or 0.5 mM P at 15 dpp. Infection fronts (n = 14–31) were randomly chosen from three plants in each treatment and were photographed at 4.5 to 5, 6 to 6.5, 8 to 8.5, and 24 to 24.5 h after water treatment and at 5 to 5.5, 6.5 to 7.5, 8.5 to 9.5, and 24.5 to 25.5 h after P treatment, and the ratios of arbuscular developmental stages were analyzed according to the localization patterns of GFP-AM42. Bars = 20 µm in A and 50 µm in B and C.
Branched Arbuscules Are Metabolically Active
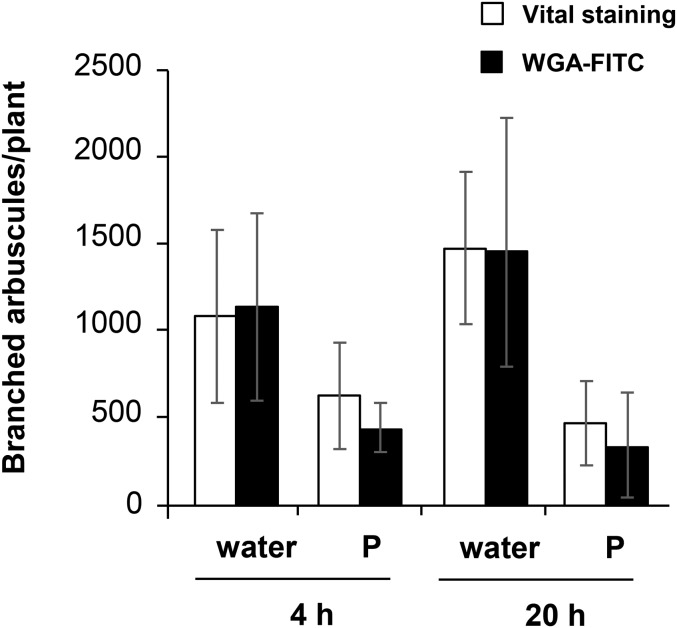
To confirm the intactness of branched arbuscules during P inhibition, metabolic activity of branched arbuscules was investigated. First, rice roots colonized with R. irregularis were treated with water or 0.5 mM P solution at 14 dpp, and the roots were subjected to fungal vital staining (MacDonald and Lewis, 1978) or WGA-FITC staining at 4 or 20 h after the treatments. The number of colonized regions was similar between vital staining and WGA-FITC staining irrespective of time or treatment (Supplemental Fig. S5A). Next, the number of branched arbuscules per colonized region was analyzed. Branched arbuscules stained with vital staining were determined according to a criterion described previously (Kobae et al., 2014). The number of branched arbuscules was decreased by P treatment, whereas the values were not significantly different between vital staining and WGA-FITC staining (Supplemental Fig. S5B). The total number of branched arbuscules per plant was then determined by multiplying the number of the colonized regions per plant and the average value of the numbers of branched arbuscules per colonized region. The numbers of metabolically active branched arbuscules were not significantly different between water and P treatment at 4 and 20 h (Fig. 4). The data suggest that branched arbuscules were metabolically active during P inhibition at least up to 20 h after P treatment. Transmission electron microscopy analysis also indicated that AM fungi maintained their cytoplasm in arbuscule branches (Supplemental Fig. S6).
Figure 4.
Branched arbuscules are metabolically active after P treatment. Rice roots colonized with R. irregularis were treated with water or 0.5 mM Pi at 14 dpp. Roots were subjected to fungal vital staining or WGA-FITC staining at 4 or 20 h after the treatments. To calculate the total number of branched arbuscules per plant, numbers of colonized regions per plant (n = 5, biologically independent) were first determined. Then, numbers of branched arbuscules in each colonized region (n = 5, in each plant) were counted (Supplemental Fig. S5). The total numbers of branched arbuscules per plant were determined by multiplying the number of colonized regions and the average value of the numbers of branched arbuscules in colonized region. Data are presented as means ± sd. Two-way ANOVAs were performed on the data, with treatments (water/P) and staining method (WGA/vital staining) as factors for each time point separately. Staining methods had no significant effect on the number of branched arbuscules per plant, while P treatments had significant effects (P < 0.01).
The Life Span of Branched Arbuscules Is Not Affected by P Treatment
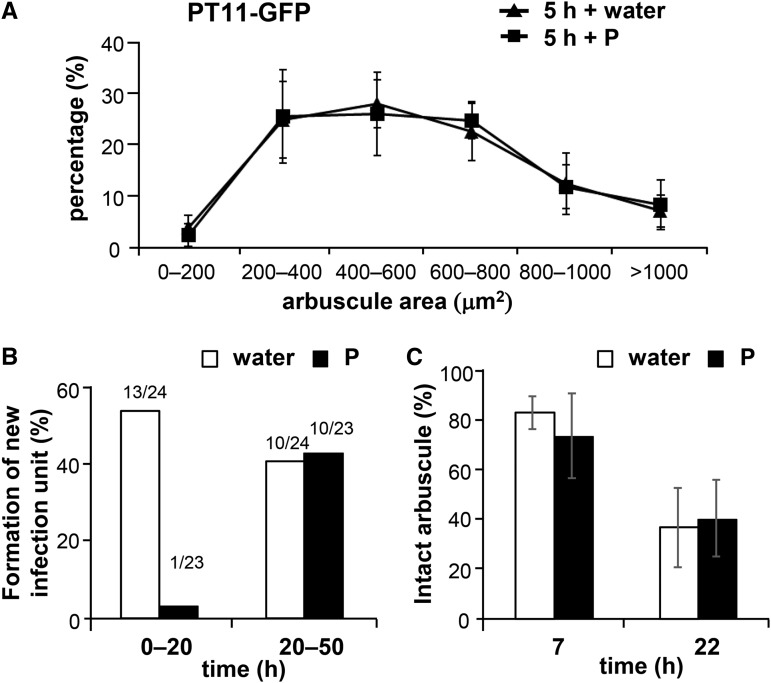
To further confirm the stability of branched arbuscules during P inhibition, arbuscule sizes were analyzed using the GFP-fused rice phosphate transporter PT11, which is specifically located on periarbuscular membranes surrounding arbuscule branches (Kobae and Hata, 2010; Kobae and Fujiwara, 2014) and is responsible for phosphate uptake in the rice mycorrhizal pathway (Yang et al., 2012). The size of arbuscules indicated by PT11-GFP was not affected by P treatment at 5 h after the treatment (Fig. 5A). In further experiments, the in vivo inhibition of new arbuscule formation and life span of arbuscule were examined using live imaging of rice expressing PT11-GFP after treatment with water or P. The appearance and disappearance of new infection units over 72 h in the water control were observed, and new infection units emerged in 13 of 24 and 10 of 24 colonized regions during 0- to 20- and 20- to 50-h periods after water treatments, respectively (Fig. 5B). In contrast, the emergence of new infection units in P-treated roots was hardly observed (Supplemental Fig. S7) and appeared in only 1 of 23 colonized regions during the 0- to 20-h period after P treatment (Fig. 5B). However, the emergence of new infection units recovered in 10 of 23 colonized regions during the 20- to 50-h period after P treatment, which was comparable to the numbers of infection units in the water control during this period (Fig. 5B), indicating the temporary nature of P inhibition. Subsequently, we used PT11-GFP labeling to track the life span of arbuscules. In these experiments, a total of 73 and 71 branched arbuscules were randomly chosen from water- and P-treated roots, respectively, and their presence at 7 and 22 h after treatment did not differ between water- and P-treated roots (Fig. 5C), suggesting the stability of the life span of branched arbuscules during P inhibition.
Figure 5.
PT11-GFP-stained branched arbuscules are resistant to P inhibition. A, Size distribution of arbuscules indicated by the P transporter PT11-GFP. proPT11-PT11-GFP roots were colonized with R. irregularis (14 dpp) and were then treated with water or 0.5 mM P for 5 h in pot culture. Five colonized regions were randomly chosen from each of three plants, and the areas of longitudinal optical sections of all visible arbuscules (40–126 arbuscules from each colonized region) were measured. B, The frequency of new infection unit developments after water or P treatment. Colonized regions (24, water treatment; 23, P treatment) were tracked for 50 h using live imaging, and the emergence of new infection units after 0 to 20 h and 20 to 50 h were analyzed. Values above columns indicate the ratios of the numbers of colonized regions with new infection units and numbers of colonized regions observed. C, The comparison of arbuscule life spans between water and P treatment. A total of 73 and 71 arbuscules were randomly chosen in six colonized regions from two plants treated with water and in eight colonized regions from three P-treated plants, respectively, and were tracked using live imaging. The numbers of intact arbuscules in each colonized region before treatment with water or P were defined as 100%, and the percentages of intact arbuscules after 7 and 22 h are shown. Data are presented as means ± sd. Bars = 100 µm in A and B.
PT11 Is Required for Stabilization of Branched Arbuscules during P Inhibition
Proper localization of symbiosis-specific P transporter on periarbuscular membrane is crucial for the normal development of arbuscule branches (Pumplin et al., 2012). As immature arbuscule-containing cells lack symbiosis-specific P transporter (Kobae and Hata, 2010; Pumplin et al., 2012) and PT11-GFP-positive arbuscules are resistant to P inhibition (Fig. 5), we considered the possibility that the presence of PT11 is related to the stability of branched arbuscules. To evaluate this hypothesis, we analyzed the arbuscule density of the rice pt11-1 mutant after P treatment. Under low P condition, arbuscule density of pt11-1 mutant was comparable to that of the wild type at 14 dpp (Fig. 6), and no obvious difference was observed. When pt11-1 or wild-type plants colonized with R. irregularis (14 dpp) were transplanted into pots together with germinated acceptor wild-type seeds, the numbers of colonized regions formed in the acceptor roots were not significantly different at 14 d post transplant (Supplemental Fig. S8A), but the length of colonized regions in the acceptor roots inoculated with pt11-1 was reduced compared to those inoculated with the wild type (Supplemental Fig. S8B). The data suggest that fungal colonization is maintained, but the fungal growth is not sustained in pt11-1 mutant. Despite normal arbuscule density of pt11-1 roots under low P condition, the arbuscule density was significantly reduced after P treatments compared with that of the wild type (Fig. 6), indicating that PT11 is required for arbuscule stability during P inhibition.
Figure 6.
Arbuscule density in the roots of pt11-1 is significantly reduced by P supply compared with the wild type. The roots of the wild type or pt11-1 mutant colonized with R. irregularis were treated with water or 0.5 mM Pi at 14 dpp for 5 h in pot culture. The roots were stained with WGA-FITC to detect the intraradical mycelium. Colonized regions (n = 60) were randomly chosen from six plants, and the longitudinal lengths and numbers of arbuscules with fine branches in each colonized region were measured. Young arbuscules with only trunk or senescent arbuscules that were sparsely stained with WGA-FITC were not included. Middle lines of box plots represent median values, and bars represent ranges (minimum to maximum). Two-way ANOVAs were performed on the data, with genotype (wild type/pt11-1) and treatment (water/phosphate) as factors. Both genotype (P < 0.01) and treatment (P < 0.05) had significant effects. There was no significant interaction between genotype and treatment. **P < 0.01, Welch’s t test (water versus P); ns, no significant difference.
Vesicle Formation Is Increased by P Treatment
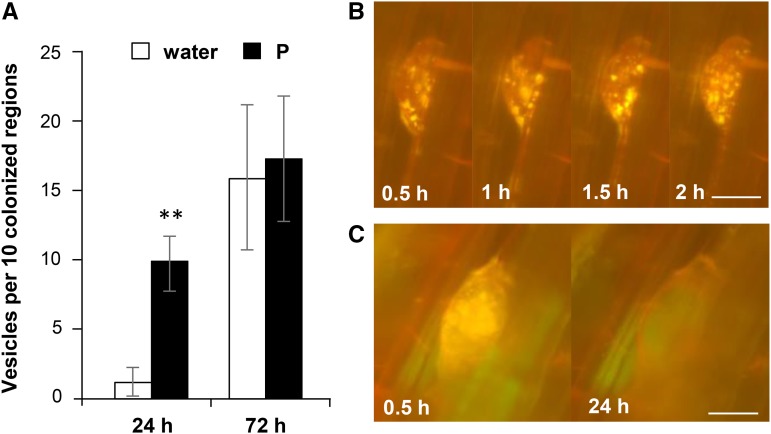
WGA-FITC staining of mycorrhizal roots at 24 h after P treatment revealed 7-fold increases in the numbers of vesicles, whereas the numbers of vesicles were similar at 72 h after P or water treatments (Fig. 7A). Subsequently, lipid droplets were detected in vesicles using the fluorescent probe Nile red (NR) in live imaging analyses of PT11-GFP rice. NR is a hydrophobic probe for neutral lipids, = emits yellow-red fluorescence (Greenspan et al., 1985), and can penetrate the cortex of rice roots within 30 min (Kobae et al., 2014). Accumulation of lipid droplets was observed in vesicles at 30 min after NR treatment and changed every minute of the 2-h observation period, suggesting the mobility of lipid droplets in vesicles (Fig. 7B). Whether the lipid droplets increased more rapidly after high P treatment compared with water treatment was unclear. However, some vesicles lost their lipid droplets during observation (Fig. 7C). To determine whether quenching or dilution of NR in roots or mycelium compromised detection in lipid droplets, live imaging was performed with additional NR, and lipid accumulation was monitored. In these experiments, no increases in lipid droplet accumulation were observed at 30 min after NR treatment, whereas numerous lipid droplets accumulated after 1 d (Supplemental Fig. S9). Taken together, these observations confirm that lipid droplets accumulated and then decreased in vesicles within a few days.
Figure 7.
Vesicle formation is increased in P-treated plants. A, Vesicle numbers. Rice plants were colonized with R. irregularis, treated with water or 0.5 mM P at 14 dpp, and stained with WGA-FITC. The numbers of vesicles in 10 randomly chosen colonized regions from three plants were measured after 24 and 72 h. Data are presented as means ± sd of three biological replicates. **P < 0.01; Welch’s t test (water versus P). B, Live imaging of a vesicle with accumulated lipid droplets. proPT11-PT11-GFP roots were colonized with R. irregularis in a live imaging system, treated with P at 13 dpp, and supplemented with NR at 15 dpp, and live imaging was performed 60 min after the addition of NR. Four time points (0.5, 1, 1.5, and 2 h from the start of time lapse) are shown. C, Live imaging of a vesicle that lost lipid droplets. Two time points (0.5 and 24 h from the start of time lapse) are shown. Bars = 20 µm in B and C.
DISCUSSION
The stage of P inhibition during the growth of infection units was previously uncharacterized. In this study, P inhibition primarily occurred during the development of new arbuscules that lack branches, and other intraradical colonization processes were not inhibited. Specifically, older arbuscules with PT11-GFP-labeled branches were tolerant to P inhibition and showed robust life cycles and intraradical accommodation of AM fungal infections under these conditions. Although P reportedly reduced numbers of vesicles, our data indicate that vesicle formation was induced under conditions of P inhibition, suggesting a novel regulatory mode of intraradical colonization that is mycelium-type specific in short-term effects of P inhibition.
P Treatment Selectively Inhibits the Development of New Arbuscules
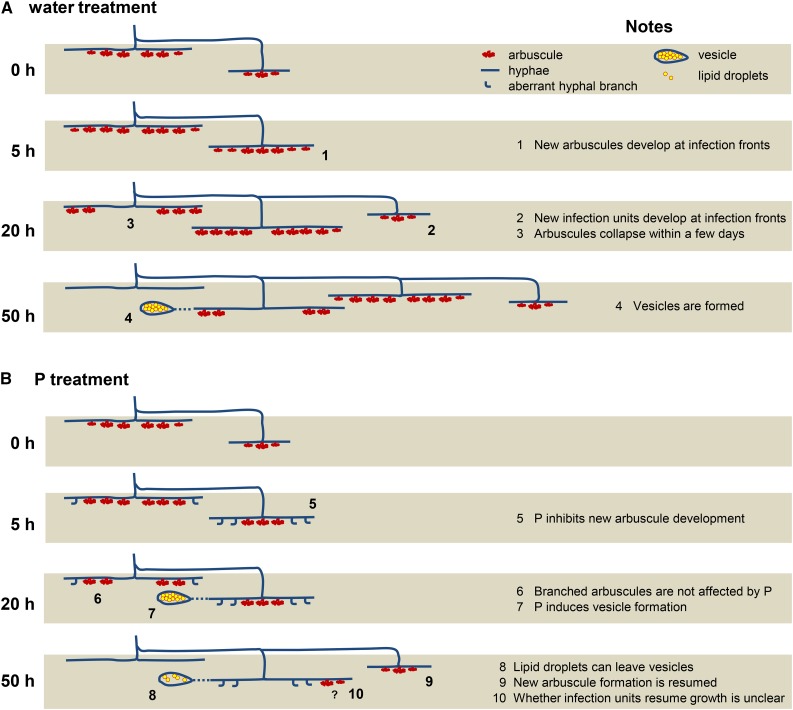
Under conditions of low P, continuous intraradical colonization is achieved through the repetitive developments of new infection units (Fig. 8A). In agreement, infection units and arbuscules reportedly senesce after the cessation of the intercellular longitudinal extension of hyphae (Cox and Sanders, 1974; Kobae and Hata, 2010). Moreover, recent live imaging of mycorrhizal roots of rice showed that colonized cells tend to not be recolonized as readily as previously uncolonized cells. Therefore, well-colonized cortical cells may support continuous cycles of infection unit formation (Kobae and Fujiwara, 2014). In this study, P treatment rapidly reduced the arbuscule density of colonized regions and increased the number of immature arbuscules at infection fronts after 5 h of P treatment (Fig. 8B). Because the longitudinal length of colonized regions and the density of hyphopodia were not changed at 20 h after P treatment, the reduced density of arbuscules during the early stages of P inhibition may reflect the inhibition of new arbuscule development. Accordingly, the lack of endomembrane localization of GFP-AM42 presentation at infection fronts indicated the inhibition of normal membrane trafficking, whereas preformed arbuscules were tolerant to P treatments. After P treatment, (1) size of branched arbuscules indicated by fungal cell wall staining and PT11-GFP imaging was not changed, (2) intracellular localization patterns of PT11-GFP and GFP-AM42 were not changed in cells containing branched arbuscules, (3) metabolic activities of branched arbuscules were maintained, and (4) live imaging of PT11-GFP indicated no changes in the life spans of branched arbuscules. Thus, reduced arbuscule density does not reflect the early degeneration of branched arbuscules. The stability of older arbuscules against P inhibition was also suggested by other reports: P uptakes via AM fungi in the mycorrhizal roots of wheat (Triticum aestivum) and tomato (Solanum lycopersicum) treated with P were maintained, whereas colonization rates decreased (Li et al., 2006; Nagy et al., 2009). Breuillin et al. (2010) reported that P did not reduce the colonization level of petunia mycorrhizal roots before 2 weeks after P supply, but the transcript levels of symbiotic P transporter genes were decreased within 2 d. It is possible that symbiotic P transporter proteins localize periarbuscular membranes to maintain arbuscules during P inhibition, but the transcription is immediately suppressed.
Figure 8.
Diagram of P inhibition in mycorrhizal rice roots. A, Diagram of the development of intraradical colonization under conditions of low P. (1) Young infection units grow and develop new arbuscules at infection fronts. (2) Colonized regions develop through the repetitive formation of infection units, and new infection units develop immediately adjacent to established infection units. (3) Arbuscules collapse from near the hyphopodia because of their short and constant life span. (4) Colonized regions with senescent arbuscules often form vesicles, but the precise timing and localization cannot be predicted (represented by the dotted line). B, Diagram of the development of intraradical colonization under conditions of high P. (5) P treatment induces aberrant hyphal branching at infection fronts. New infection units hardly develop within colonized regions for at least 1 d after P treatment. The inhibition of new arbuscule formation eventually reduces the density of arbuscules. (6) P does not affect the morphology or life span of preformed branched arbuscules. (7) P treatment increases the formation of vesicles within 1 d and lipid droplets accumulate. (8) Accumulated lipid droplets can leave vesicles. (9) New colonization is resumed 2 d after P treatment suggesting that P inhibition is temporary. (10) It remains unclear whether aberrant branches become arbuscules or whether infection units resume growth.
Vesicle Formation Is Induced during P Inhibition
Our observations also revealed that P treatment increased the formation of vesicles in roots (Fig. 8B). The precise biological roles of vesicles remain unclear (Smith and Read, 2008). Early cytological studies of vesicles suggested that the protoplasm of young vesicles contains many nuclei, glycogen granules, and small vacuoles, while mature vesicles contain many lipid droplets (Bonfante-Fasolo, 1984). Given that the number of vesicles often increase in old or dead roots (Bonfante-Fasolo, 1984), vesicles are thought to be resting organs, playing a significant role as propagules within roots (Smith and Read, 2008). However, to our knowledge, rapid changes in the number or the content of vesicle lumen in response to environmental factor have not been described. We demonstrated that lipid droplets labeled with NR in vesicles decreased within a few days, and colonization resumed between 20 and 50 h after P treatment. The mobility of lipid droplets in vesicles is consistent with previous reports that indicate the inconsistent timing of vesicle formation and accumulation of neutral lipids (Graham et al., 1995; van Aarle and Olsson, 2003; Olsson et al., 2010). McLennan (1926) observed that swelling vesicles are highly protoplasmic and contain many lipids in the mycorrhizal roots of Lolium temulentum (ryegrass). At a later stage of colonization, lipids are removed from them and rapidly carried to the hyphae of subsequent colonization. The vesicles consequently become empty; thus, vesicles can be regarded as temporary reserve organs (McLennan, 1926). Our observation is consistent with the opinion of McLennan (1926), and the variable vesicle contents may mirror the pleiotropic roles of vesicles in the colonization cycle of mycorrhizal roots. Although further studies will be needed, it is tempting to speculate that vesicles serve as, during P inhibition, a “retention basin” that sequestrates the fungal cytoplasm during P inhibition. Arbuscules collapse within a few days even under low P conditions, likely leading to the withdrawal of cytoplasmic and structural constituents (Kobae et al., 2014). Accordingly, in a previous study on rice mycorrhizal roots, the collapse of 30 arbuscules leads to withdrawals of fungal cytoplasm volumes that are equivalent to the volume of six to seven plant cells (Kobae et al., 2014). Because secondary infection units are reportedly fueled from previously formed infection units (Sanders and Sheikh, 1983) and, in this study, the development of new infection units were inhibited after P treatment, withdrawn fungal cytoplasm from collapsed arbuscules may lose their destinations. Therefore, cytoplasm and lipid droplets from collapsed arbuscules (Kobae et al., 2014) may be sequestered into vesicles during P inhibition.
Short-Term P Inhibition versus Long-Term P Inhibition
In contrast with our observations, early studies showed that P inhibition decreases the numbers of vesicles (Abbott and Robson, 1979; Stribley et al., 1980; Abbott et al., 1984; Amijee et al., 1989: Bruce et al., 1994) and lengths of colonized regions (Sanders and Tinker, 1973; Jasper et al., 1979; Buwalda et al., 1982; Graham and Leonard, 1982; Smith, 1982; Amijee et al., 1989; Miranda et al., 1989; Smith and Gianinazzi-Pearson, 1990; Braunberger et al., 1991; Thomson et al., 1991; Baon et al., 1992; Bruce et al., 1994; Tawaraya et al., 1994). However, reduced intraradical fungal colonization was observed at 8 to 108 d after the application of large quantities of P (average 231 mg/kg soil in maximum treatment; 4.8 mg/kg soil in this study) or frequent P treatments in these studies. Hence, P treatment is likely to decrease the growth of intraradical mycelium and decrease the length of extraradical hyphae (Abbott et al., 1984), which potentially contribute to subsequent infections that support repetitive colonization and further mycorrhizal development (Sanders and Sheikh, 1983; Schwab et al., 1983). Therefore, long-term P treatment likely decreases colonization by attenuating the cycle sequences of intraradical colonization as an indirect effect of primary P inhibition (Abbott et al., 1984; Bruce et al., 1994). In contrast, the numbers of vesicles and lengths of colonized regions were assessed at 1 d after P treatment in this study, representing the earliest measurements of P inhibition effects to date. We showed that cellular responses that inhibit the development of new arbuscules occurred within hours of P treatment. Differential expression of thousands of rice genes was observed in shoots and roots within 1 h after P treatment (Secco et al., 2013), further indicating molecular and cellular responses that inhibit new arbuscule development within a few hours. Intriguingly, colonization can recover within a couple of days. The soil used in this study is volcanic lapillus that strongly captures P; thus, treated P might immediately become unavailable for roots, leading to the recovery of colonization.
There is no direct evidence linking temporary P inhibition with the significant reduction of colonization levels during intensive P fertilization in the field. Long term, sequential analyses of P inhibition would help to address this question and we monitored the fate of temporary P inhibition by means of a time-course analysis of arbuscule development after P treatment. However, in this culture system, colonization is suppressed a week after the beginning of infection probably due to the spatial limitation of the culture system or malnutrition, making it difficult to characterize the colonization sequences during long-term P inhibition; therefore, it will be necessary to pursue these studies in another way.
The Potential Mechanism of P Inhibition
The molecular basis of P inhibition remains unclear (Carbonnel and Gutjahr, 2014). Transcriptome analyses of mycorrhizal petunia roots under varying P conditions demonstrated the absence of defense responses and significant down-regulation of genes encoding enzymes of carotenoid and strigolactone biosynthesis (Breuillin et al., 2010). Biosynthesis, transport, and signaling events of strigolactone-related molecules are required for normal colonization (Floss et al., 2008; Vogel et al., 2010; Kretzschmar et al., 2012; Yoshida et al., 2012; Foo et al., 2013; Gutjahr et al., 2015). Strigolactones also induce hyphal branching (Akiyama et al., 2005) and increase the production of symbiotic fungal signals (Genre et al., 2013). However, normal arbuscules are formed in plant mutants that are defective in strigolactone biosynthesis genes (Gutjahr et al., 2012), suggesting that reduced strigolactone levels are not involved in the early stages of P inhibition. Moreover, our observations suggest that P treatment interrupts the development of arbuscules at the coarse hyphal branch stage. These data are in accordance with the “birdsfoot” arbuscules described by Gutjahr and Parniske (2013), which are observed in cells with mutant or RNA interference against STR1 (Zhang et al., 2010; Gutjahr et al., 2012; Kojima et al., 2014), PAM1 (Feddermann et al., 2010), RAM2 (Wang et al., 2012), VAMP72s (Ivanov et al., 2012), SYP132A (Pan et al., 2016), EXO70I (Zhang et al., 2015), VTI12 (Lota et al., 2013), ERF1 (Devers et al., 2013), RED (Groth et al., 2013; Gutjahr and Parniske, 2013), RAM1/ATA (Rich et al., 2015), RAD1 (Park et al., 2015; Xue et al., 2015), and FatM (Bravo et al., 2016). Hence, P inhibition may be related to the functions of these genes. Recently, it has been shown that RAM1, a GRAS-type transcription factor, regulates the expressions of at least STR and EXO70I, which are required for the support of arbuscule branching (Park et al., 2015). RAM1 expression is also regulated by DELLA/gibberellin signaling module, and DELLA level is regulated by P status (Floss et al., 2013; Takeda et al., 2015; Park et al., 2015). We showed that the numbers of GFP-AM42-positive young arbuscules in infection fronts of P-treated roots tended to be decreased compared to those of water-treated roots, suggesting that the development of arbuscules was arrested at their young stages. Potentially, transcriptional regulation of genes responsible for the development of arbuscules may be suppressed during P inhibition.
In this study, we show that P treatment selectively inhibits the development of immature arbuscules, and branched arbuscules are tolerant to P inhibition. Potentially, the selectivity of P inhibition indicates the presence of a localized stabilization mechanism of branched arbuscules. Considering that (1) the loss of periarbuscular membrane localization of the M. truncatula PT11 ortholog MtPT4S115F led to the premature degeneration of arbuscules (Pumplin et al., 2012), (2) PT11/PT4 is not localized to the plasma membrane or membranes surrounding young arbuscules that have no fine branches (Harrison et al., 2002; Pumplin and Harrison, 2009; Kobae and Hata, 2010), and (3) plants can recognize locally increased P supply, which stimulate increased carbon allocation to a localized regions around the P supply (Drew, 1975; Fitter, 2006), it was hypothesized that unbranched immature arbuscules may not contribute to P uptake and that the appropriate P uptake at branched arbuscules may be related to the stabilization of arbuscules. Consistent with this, pt11-1 was more sensitive to P inhibition than that of the wild type, suggesting that P uptake through PT11 in colonized cells stabilizes arbuscules during P inhibition. Alternatively, PT11 in arbuscule-containing cells may act as a component of P-sensing machinery as proposed in the root tips of M. truncatula and Lotus japonicas (Volpe et al., 2016).
It has been shown that long-term P inhibition is partially suppressed under low nitrogen (N) conditions (Blanke et al., 2005; Nouri et al., 2014). Furthermore, premature arbuscule degeneration in M. truncatula pt4 mutant is also suppressed under low N conditions (Javot et al., 2011), and an ammonium transporter of periarbuscular membrane is implicated in this regulation (Breuillin-Sessoms et al., 2015). Thus, it can be assumed that plant N status may control the conditions for the maintenance of arbuscules through the transport/signaling function of symbiotic N transporters in mature arbuscule-containing cells (Breuillin-Sessoms et al., 2015). In this study, arbuscule development of pt11-1 mutant was normal at least up to 14 dpp under low P condition, suggesting that pt11-1 in this growth condition was basically low N status and was stabilized by the mechanism of “N-mediated maintenance.” In fact, pt11-1 plants were grown under semiarid condition and were supplied with no nutrition (except for P) throughout the experiments. Alternatively, the pt11/pt4 phenotype (i.e. accelerated arbuscule turnover) may not be detected at early time points (Javot et al., 2007). Nevertheless, P inhibition was accelerated in the pt11-1 mutant, indicating that “P-mediated maintenance” is crucial for the stabilization of arbuscules during P inhibition. In addition, nurse pt11-1 plant was not able to support the growth of fungus even if the arbuscule formation is maintained, suggesting that PT11 is implicated in the maintenance of life cycle of AM fungus. Taken together, the data highlight a novel role of symbiotic P transporter in supporting arbuscule maintenance at high P conditions that interfere with arbuscule development.
In conclusion, our data strongly indicate that P treatment inhibited the development of new arbuscules within 5 h and that this inhibition was temporary. In contrast to assumptions derived from long-term P treatments, vesicle formation was enhanced by P treatment in the short term, suggesting that mycorrhizal roots assume a resting state but continue to accommodate AM fungi under these conditions. The robust nature of arbuscule formation would guarantee continuous P uptake during growth in conditions of varying P fertility, such as those observed in the natural ecosystems. Moreover, we demonstrated that the stability of mature arbuscules under high P condition is PT11 dependent, revealing a novel function of the P transporter for the stable colonization of AM fungi.
MATERIALS AND METHODS
Plant Growth and P Treatment in Pot Culture
Rice seeds (Oryza sativa cv Nipponbare) were surface sterilized with bleach (2.5% available chloride) for 5 min, rinsed with excess deionized water five times, and immersed in deionized water for 2 d at 28°C. The germinated seeds were grown singly in 100-mL pots (D-100; Teraoka). The soil consists of 20 g (bottom layer) of Akadama soil (tuff loam; Setogahara Kaen) and 45 g (upper layer) of Kanuma soil (weathered volcanic lapillus)/Ezo sand (small pumice)/Nippi soil (granular potting soil; Nihon Hiryo) mixture (6:2:1, by weight). Plants were inoculated with Rhizophagus irregularis (Premier Tech) by mixing 500 spores throughout the upper soil mixture before planting the germinated seeds. Plant pots were placed in a flat-bottom tray and arranged randomly in a greenhouse under 15-h-light/9-h-dark photoperiods (26–30°C/23–25°C). No other nutrients were added except Nippi soil, and water was supplied from the bottom by maintaining a water level up to 5 mm in depth. Twenty milliliters of P solution (0.5 mm NaH2PO4/Na2HPO4, pH 5.4) was added from the bottom of each plant (4.8 mg P/kg soil). Control pots received the same volume of water.
Plant Growth and P Treatment in Live Imaging System
Germinated seeds of proPT11-PT11-GFP (PT11-GFP rice; Kobae and Hata, 2010) were planted to 90 mm (diameter) × 15 mm (height) petri dishes of a live imaging system (Kobae and Fujiwara, 2014). The soil of the live imaging system consists of 31 g of Kanuma soil/Ezo sand/Nippi potting soil mixture (20:8:3, by weight). Water was supplied from the bottom by capillary action of a 20-mm-wide unwoven cloth. The base of the dish incorporated a 50- × 20-mm rectangular window covered with a 55- × 25-mm coverslip. Five hundred spores of R. irregularis with 2 g of Kanuma soil were laid on the coverslip. Petri dishes were placed on the inverted platform of a Zeiss epifluorescence microscope (Axio observer A1). Three milliliters of 5 mm P solution (15 mg P/kg soil) was applied to the soil surface through two holes (5 mm in diameter) on the lid of the petri dish. Considering the possibility that P applied from the top is caught at soil surface (volcanic lapillus), the amount of P applied was 3 times higher than that of pot experiments. Control dishes received the same volume of water.
Fungal Cell Wall Staining
Plants were extracted carefully from the pot. Roots were washed free of soil using water and detached from the shoots. Roots were fixed in 50% ethanol for 2 h, cleared in 20% (w/v) KOH for 2 d at room temperature, washed five times with PBS (135 mm NaCl, 25 mm KCl, and 10 mm Na2HPO4, pH 7.5), and then soaked in PBS containing 0.2 µg mL−1 WGA-FITC (Vector Laboratories). The roots were kept in the same solution at least for 16 h in dark, washed with PBS once, and then observed.
Microscopy
Colonized sites were located and dissected using a fluorescence stereomicroscope (Leica M165FC) and then analyzed using an epifluorescence microscope (Zeiss Axio observer A1) or a confocal laser scanning microscope (Olympus FV1000). GFP-AM42 or PT11-GFP fluorescence images were analyzed within 10 min of root excision. In live imaging, PT11-GFP rice seedlings grown in 90-mm petri dishes were placed on the inverted platform of a Zeiss Axio observer A1. Images were processed using ZEN2011 (Zeiss). Rice seedling leaves were kept illuminated at 25°C under continuous light with a portable fluorescent lamp during live imaging. The lengths of colonized region, the sizes of arbuscules were measured using ImageJ.
Electron Microscopy
Twenty milliliters of 0.5 mM P solution was added to the pots of PT11-GFP rice from the bottom at 14 dpp. Control pots received the same volume of water. Colonized root regions that express PT11-GFP were dissected (<5 mm) using a fluorescence stereomicroscope (Leica M165FC). The root segments were immediately fixed in 2% glutaraldehyde in 20 mm sodium cacodylate buffer, pH 7.4, for 2 h on ice and then washed four times for 20 min each in 20 mm sodium cacodylate buffer, pH 7.4, and postfixed in 2% OsO4 for 2 h. The root segments were dehydrated through an ethanol series and then through propylene oxide series. The root segments were then infiltrated with modified Spurr’s resin, combination with Quetol 653 (Nisshin EM; Kushida, 1980), gradually increased concentration 50, 75, and 100% for 1 d each, and polymerized at 70°C for 2 d. Ultrathin sections (50–90-nm thick) were cut, stained with uranyl acetate and lead citrate, and observed by a transmission electron microscope (JEM1010; JEOL).
Fungal Vital Staining
Fungal viability was assessed by vital staining, which detects in situ succinate dehydrogenase activity with nitroblue tetrazolium (NBT; MacDonald and Lewis, 1978). The whole root system was stained as described previously (Kobae et al., 2014). GFP-AM42 (Kobae and Fujiwara, 2014) positive roots were dissected using a fluorescent stereomicroscope, and the root segments were incubated at room temperature for 30 min in the dark in NBT solution containing 50 mm Tris-HCl buffer (pH 7.4), 1 mg mL−1 NBT, 0.5 mm MgCl2, and 250 mm sodium succinate.
Lipid Droplet Staining
NR (AnaSpec) was diluted in deionized water or 0.5 mM P solution (8 mg mL−1) and immediately applied to the soil of live imaging system as described previously (Kobae et al., 2014).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. The measurement of the lengths of hyphal-colonized root regions.
Supplemental Figure S2. Confocal laser scanning microscope image of arbuscule trunks/undeveloped arbuscules.
Supplemental Figure S3. P treatment does not change colonization levels at 20 h.
Supplemental Figure S4. No changes in localization patterns of GFP-AM42 are observed in cells with finely branched arbuscules.
Supplemental Figure S5. Comparison of the colonization levels evaluated by vital staining or WGA-FITC staining.
Supplemental Figure S6. AM fungi maintained their cytoplasm in arbuscule branches in P-treated roots.
Supplemental Figure S7. New infection units are hardly developed in colonized region during P inhibition.
Supplemental Figure S8. pt11-1 nurse plant inoculation assay.
Supplemental Figure S9. Lipid droplets accumulate and decrease in vesicles.
Supplementary Material
Acknowledgments
We thank Katsuharu Saito and Tatsuhiro Ezawa for critical reading of the manuscript. We thank Uta Paszkowski for providing the pt11-1 mutant. We thank Hiroshi Moriyama (Nihon Hiryo Co.) for providing Nippi soil.
Glossary
- AM
arbuscular mycorrhizal
- dpp
days post-planting
- NR
Nile red
- WGA-FITC
fluorescein isothiocyanate conjugated to wheat germ agglutinin
- NBT
nitroblue tetrazolium
References
- Abbott LK, Robson AD (1979) A quantitative study of the spores and anatomy of mycorrhizas formed by a species of Glomus, with reference to its taxonomy. Aust J Bot 27: 363–375 [Google Scholar]
- Abbott LK, Robson AD, De Boer G (1984) The effect of phosphorus on the formation of hyphae in soil by the vesicular-arbuscular mycorrhizal fungus, Glomus fasciculatum. New Phytol 97: 437–446 [Google Scholar]
- Akiyama K, Matsuzaki K, Hayashi H (2005) Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature 435: 824–827 [DOI] [PubMed] [Google Scholar]
- Amijee F, Tinker PB, Stribley DP (1989) The development of endomycorrhizal root systems, VII. A detailed study of the effects of soil phosphorus on colonization. New Phytol 111: 435–446 [DOI] [PubMed] [Google Scholar]
- Balzergue C, Chabaud M, Barker DG, Bécard G, Rochange SF (2013) High phosphate reduces host ability to develop arbuscular mycorrhizal symbiosis without affecting root calcium spiking responses to the fungus. Front Plant Sci 4: 426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balzergue C, Puech-Pagès V, Bécard G, Rochange SF (2011) The regulation of arbuscular mycorrhizal symbiosis by phosphate in pea involves early and systemic signalling events. J Exp Bot 62: 1049–1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baon JB, Smith SE, Alston AM, Wheeler RD (1992) Phosphorus efficiency of three cereals as related to indigenous mycorrhizal infection. Aust J Agric Res 43: 479–491 [Google Scholar]
- Baylis GTS. (1967) Experiments on the ecological significance of phycomycetous mycorrhizas. New Phytol 66: 231–243 [Google Scholar]
- Bieleski RL. (1973) Phosphate pools, phosphate transport, and phosphate availability. Annu Rev Plant Physiol 24: 225–252 [Google Scholar]
- Blanke V, Renker C, Wagner M, Füllner K, Held M, Kuhn AJ, Buscot F (2005) Nitrogen supply affects arbuscular mycorrhizal colonization of Artemisia vulgaris in a phosphate-polluted field site. New Phytol 166: 981–992 [DOI] [PubMed] [Google Scholar]
- Bonfante-Fasolo P. (1984) Anatomy and morphology of VA mycorrhizae. In Powell CL, Bagyaraj DJ, eds, VA Mycorrhizas. CRC Press; , Boca Raton, FL, pp: 5–33 [Google Scholar]
- Bonfante-Fasolo P, Faccio A, Perotto S, Schubert A (1990) Correlation between chitin distribution and cell wall morphology in the mycorrhizal fungus Glomus versiforme. Mycol Res 94: 157–165 [Google Scholar]
- Braunberger PG, Millers MH, Peterson RL (1991) Effect of phosphorus nutrition on morphological characteristics of vesicular arbuscular mycorrhizal colonization of maize. New Phytol 119: 107–113 [DOI] [PubMed] [Google Scholar]
- Bravo A, York T, Pumplin N, Mueller LA, Harrison MJ (2016) Genes conserved for arbuscular mycorrhizal symbiosis identified through phylogenomics. Nat Plants; 2: 15208. [DOI] [PubMed] [Google Scholar]
- Breuillin F, Schramm J, Hajirezaei M, Ahkami A, Favre P, Druege U, Hause B, Bucher M, Kretzschmar T, Bossolini E, et al. (2010) Phosphate systemically inhibits development of arbuscular mycorrhiza in Petunia hybrida and represses genes involved in mycorrhizal functioning. Plant J 64: 1002–1017 [DOI] [PubMed] [Google Scholar]
- Breuillin-Sessoms F, Floss DS, Gomez SK, Pumplin N, Ding Y, Levesque-Tremblay V, Noar RD, Daniels DA, Bravo A, Eaglesham JB, et al. (2015) Suppression of arbuscule degeneration in Medicago truncatula phosphate transporter4 mutants is dependent on the ammonium transporter 2 family protein AMT2;3. Plant Cell 27: 1352–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce A, Smith SE, Tester M (1994) The development of mycorrhizal infection in cucumber: effects of P supply on root growth, formation of entry points and growth of infection units. New Phytol 127: 507–514 [Google Scholar]
- Buwalda JG, Ross GJS, Stribley DP, Tinker PB (1982) The development of endomycorrhizal root systems IV. The mathematical analysis of the effects of phosphorus on the spread of vesicular-arbuscular mycorrhizal infection in root systems. New Phytol 92: 391–399 [Google Scholar]
- Buwalda JG, Stribley DP, Tinker PB (1984) The development of endomycorrhizal root systems. V, The detailed pattern of development of infection and the control of infection level by host in young leek plants. New Phytol 96: 411–427 [Google Scholar]
- Carbonnel S, Gutjahr C (2014) Control of arbuscular mycorrhiza development by nutrient signals. Front Plant Sci 5: 462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox G, Sanders F (1974) Ultrastructure of the host–fungus interface in a vesicular-arbuscular mycorrhiza. New Phytol 73: 901–912 [Google Scholar]
- Devers EA, Teply J, Reinert A, Gaude N, Krajinski F (2013) An endogenous artificial microRNA system for unraveling the function of root endosymbioses related genes in Medicago truncatula. BMC Plant Biol 13: 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew MC. (1975) Comparison of the effects of a localized supply of phosphate, nitrate, ammonium and potassium on the growth of the seminal root system, and the shoot, in barley. New Phytol 75: 479–490 [Google Scholar]
- Feddermann N, Muni RR, Zeier T, Stuurman J, Ercolin F, Schorderet M, Reinhardt D (2010) The PAM1 gene of petunia, required for intracellular accommodation and morphogenesis of arbuscular mycorrhizal fungi, encodes a homologue of VAPYRIN. Plant J 64: 470–481 [DOI] [PubMed] [Google Scholar]
- Fitter AH. (2006) What is the link between carbon and phosphorus fluxes in arbuscular mycorrhizas? A null hypothesis for symbiotic function. New Phytol 172: 3–6 [DOI] [PubMed] [Google Scholar]
- Floss DS, Hause B, Lange PR, Küster H, Strack D, Walter MH (2008) Knock-down of the MEP pathway isogene 1-deoxy-D-xylulose 5-phosphate synthase 2 inhibits formation of arbuscular mycorrhiza-induced apocarotenoids, and abolishes normal expression of mycorrhiza-specific plant marker genes. Plant J 56: 86–100 [DOI] [PubMed] [Google Scholar]
- Floss DS, Levy JG, Lévesque-Tremblay V, Pumplin N, Harrison MJ (2013) DELLA proteins regulate arbuscule formation in arbuscular mycorrhizal symbiosis. Proc Natl Acad Sci USA 110: E5025–E5034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foo E, Yoneyama K, Hugill CJ, Quittenden LJ, Reid JB (2013) Strigolactones and the regulation of pea symbioses in response to nitrate and phosphate deficiency. Mol Plant 6: 76–87 [DOI] [PubMed] [Google Scholar]
- Genre A, Chabaud M, Balzergue C, Puech-Pagès V, Novero M, Rey T, Fournier J, Rochange S, Bécard G, Bonfante P, Barker DG (2013) Short-chain chitin oligomers from arbuscular mycorrhizal fungi trigger nuclear Ca2+ spiking in Medicago truncatula roots and their production is enhanced by strigolactone. New Phytol 198: 190–202 [DOI] [PubMed] [Google Scholar]
- Graham JH, Hodge NC, Morton JB (1995) Fatty acid methyl ester profiles for characterization of glomalean fungi and their endomycorrhizae. Appl Environ Microbiol 61: 58–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham JH, Leonard RT (1982) Interaction of light intensity and soil temperature with phosphorus inhibition of vesicular-arbuscular mycorrhiza formation. New Phytol 91: 683–690 [Google Scholar]
- Graham JH, Leonard RT, Menge JA (1981) Membrane-mediated decrease in root exudation responsible for phorphorus inhibition of vesicular-arbuscular mycorrhiza formation. Plant Physiol 68: 548–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenspan P, Mayer EP, Fowler SD (1985) Nile red: a selective fluorescent stain for intracellular lipid droplets. J Cell Biol 100: 965–973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groth M, Kosuta S, Gutjahr C, Haage K, Hardel SL, Schaub M, Brachmann A, Sato S, Tabata S, Findlay K, Wang TL, Parniske M (2013) Two Lotus japonicus symbiosis mutants impaired at distinct steps of arbuscule development. Plant J 75: 117–129 [DOI] [PubMed] [Google Scholar]
- Gutjahr C, Gobbato E, Choi J, Riemann M, Johnston MG, Summers W, Carbonnel S, Mansfield C, Yang SY, Nadal M, et al. (2015) Rice perception of symbiotic arbuscular mycorrhizal fungi requires the karrikin receptor complex. Science 350: 1521–1524 [DOI] [PubMed] [Google Scholar]
- Gutjahr C, Parniske M (2013) Cell and developmental biology of arbuscular mycorrhiza symbiosis. Annu Rev Cell Dev Biol 29: 593–617 [DOI] [PubMed] [Google Scholar]
- Gutjahr C, Radovanovic D, Geoffroy J, Zhang Q, Siegler H, Chiapello M, Casieri L, An K, An G, Guiderdoni E, et al. (2012) The half-size ABC transporters STR1 and STR2 are indispensable for mycorrhizal arbuscule formation in rice. Plant J 69: 906–920 [DOI] [PubMed] [Google Scholar]
- Harrison MJ, Dewbre GR, Liu J (2002) A phosphate transporter from Medicago truncatula involved in the acquisition of phosphate released by arbuscular mycorrhizal fungi. Plant Cell 14: 2413–2429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov S, Fedorova EE, Limpens E, De Mita S, Genre A, Bonfante P, Bisseling T (2012) Rhizobium-legume symbiosis shares an exocytotic pathway required for arbuscule formation. Proc Natl Acad Sci USA 109: 8316–8321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasper DA, Robson AD, Abbott LK (1979) Phosphorus and the formation of vesicular-arbuscular mycorrhizas. Soil Biol Biochem 11: 501–505 [Google Scholar]
- Javot H, Penmetsa RV, Breuillin F, Bhattarai KK, Noar RD, Gomez SK, Zhang Q, Cook DR, Harrison MJ (2011) Medicago truncatula mtpt4 mutants reveal a role for nitrogen in the regulation of arbuscule degeneration in arbuscular mycorrhizal symbiosis. Plant J 68: 954–965 [DOI] [PubMed] [Google Scholar]
- Javot H, Pumplin N, Harrison MJ (2007) Phosphate in the arbuscular mycorrhizal symbiosis: transport properties and regulatory roles. Plant Cell Environ 30: 310–322 [DOI] [PubMed] [Google Scholar]
- Kobae Y, Fujiwara T (2014) Earliest colonization events of Rhizophagus irregularis in rice roots occur preferentially in previously uncolonized cells. Plant Cell Physiol 55: 1497–1510 [DOI] [PubMed] [Google Scholar]
- Kobae Y, Gutjahr C, Paszkowski U, Kojima T, Fujiwara T, Hata S (2014) Lipid droplets of arbuscular mycorrhizal fungi emerge in concert with arbuscule collapse. Plant Cell Physiol 55: 1945–1953 [DOI] [PubMed] [Google Scholar]
- Kobae Y, Hata S (2010) Dynamics of periarbuscular membranes visualized with a fluorescent phosphate transporter in arbuscular mycorrhizal roots of rice. Plant Cell Physiol 51: 341–353 [DOI] [PubMed] [Google Scholar]
- Kojima T, Saito K, Oba H, Yoshida Y, Terasawa J, Umehara Y, Suganuma N, Kawaguchi M, Ohtomo R (2014) Isolation and phenotypic characterization of Lotus japonicus mutants specifically defective in arbuscular mycorrhizal formation. Plant Cell Physiol 55: 928–941 [DOI] [PubMed] [Google Scholar]
- Kretzschmar T, Kohlen W, Sasse J, Borghi L, Schlegel M, Bachelier JB, Reinhardt D, Bours R, Bouwmeester HJ, Martinoia E (2012) A petunia ABC protein controls strigolactone-dependent symbiotic signalling and branching. Nature 483: 341–344 [DOI] [PubMed] [Google Scholar]
- Kushida H. (1980) An improved embedding method using ERL 4206 and Quetol 653. J Electron Microsc (Tokyo) 29: 193–194 [Google Scholar]
- Li H, Smith SE, Holloway RE, Zhu Y, Smith FA (2006) Arbuscular mycorrhizal fungi contribute to phosphorus uptake by wheat grown in a phosphorus-fixing soil even in the absence of positive growth responses. New Phytol 172: 536–543 [DOI] [PubMed] [Google Scholar]
- Lota F, Wegmüller S, Buer B, Sato S, Bräutigam A, Hanf B, Bucher M (2013) The cis-acting CTTC-P1BS module is indicative for gene function of LjVTI12, a Qb-SNARE protein gene that is required for arbuscule formation in Lotus japonicus. Plant J 74: 280–293 [DOI] [PubMed] [Google Scholar]
- MacDonald RM, Lewis M (1978) The occurrence of some acid phosphatases and dehydrogenases in the vesicular–arbuscular mycorrhizal fungus Glomus mosseae. New Phytol 80: 135–141 [Google Scholar]
- Marschner H. (1995) Mineral Nutrition of Higher Plants, Ed 2 Academic Press, London [Google Scholar]
- McLennan EI. (1926) The endophytic fungus of Lolium. II. The mycorrhiza on the roots of Lolium temulentum L. with a discussion on the physiological relationships of the organism concerned. Ann Bot (Lond) 40: 43–68 [Google Scholar]
- Miranda JCC, Harris PJ, Wild A (1989) Effects of soil and plant phosphorus concentrations on vesicular arbuscular mycorrhiza in sorghum plants. New Phytol 112: 405–410 [Google Scholar]
- Mosse B. (1973) Plant growth responses to vesicular-arbuscular mycorrhiza IV. In soil given additional phosphate. New Phytol 72: 127–136 [Google Scholar]
- Nagy R, Drissner D, Amrhein N, Jakobsen I, Bucher M (2009) Mycorrhizal phosphate uptake pathway in tomato is phosphorus-repressible and transcriptionally regulated. New Phytol 181: 950–959 [DOI] [PubMed] [Google Scholar]
- Nouri E, Breuillin-Sessoms F, Feller U, Reinhardt D (2014) Phosphorus and nitrogen regulate arbuscular mycorrhizal symbiosis in Petunia hybrida. PLoS One 9: e90841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson PA, Rahm J, Aliasgharzad N (2010) Carbon dynamics in mycorrhizal symbioses is linked to carbon costs and phosphorus benefits. FEMS Microbiol Ecol 72: 125–131 [DOI] [PubMed] [Google Scholar]
- Pan H, Oztas O, Zhang X, Wu X, Stonoha C, Wang E, Wang B, Wang D (2016) A symbiotic SNARE protein generated by alternative termination of transcription. Nat Plants; 2: 15197. [DOI] [PubMed] [Google Scholar]
- Park HJ, Floss DS, Levesque-Tremblay V, Bravo A, Harrison MJ (2015) Hyphal branching during arbuscule development requires RAM1. Plant Physiol 169: 2774–2788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pumplin N, Harrison MJ (2009) Live-cell imaging reveals periarbuscular membrane domains and organelle location in Medicago truncatula roots during arbuscular mycorrhizal symbiosis. Plant Physiol 151: 809–819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pumplin N, Zhang X, Noar RD, Harrison MJ (2012) Polar localization of a symbiosis-specific phosphate transporter is mediated by a transient reorientation of secretion. Proc Natl Acad Sci USA 109: E665–E672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich MK, Schorderet M, Bapaume L, Falquet L, Morel P, Vandenbussche M, Reinhardt D (2015) The Petunia GRAS transcription factor ATA/RAM1 regulates symbiotic gene expression and fungal morphogenesis in arbuscular mycorrhiza. Plant Physiol 168: 788–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders FE, Sheikh NA (1983) The development of vesicular-arbuscular mycorrhizal infection in plant root system. Plant Soil 71: 223–246 [Google Scholar]
- Sanders FE, Tinker PB (1973) Phosphate flow into mycorrhizal roots. Pestic Sci 4: 385–395 [Google Scholar]
- Schwab SM, Menge JA, Leonard RT (1983) Comparison of stages of vesicular arbuscular mycorrhizal formation in sudangrass grown at two levels of phosphorus nutrition. Am J Bot 70: 1225–1232 [Google Scholar]
- Secco D, Jabnoune M, Walker H, Shou H, Wu P, Poirier Y, Whelan J (2013) Spatio-temporal transcript profiling of rice roots and shoots in response to phosphate starvation and recovery. Plant Cell 25: 4285–4304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S, Parniske M (2012) Activation of calcium- and calmodulin-dependent protein kinase (CCaMK), the central regulator of plant root endosymbiosis. Curr Opin Plant Biol 15: 444–453 [DOI] [PubMed] [Google Scholar]
- Smith SE. (1982) Inflow of phosphate into mycorrhizal and nonmycorrhizal Trifolium subterraneum at different levels of soil phosphate. New Phytol 90: 293–303 [Google Scholar]
- Smith SE, Gianinazzi-Pearson V (1990) Phosphate uptake and arbuscular activity in mycorrhizal Allium cepa L: effects of photon irradiance and phosphate nutrition. Aust J Plant Physiol 17: 177–188 [Google Scholar]
- Smith SE, Jakobsen I, Grønlund M, Smith FA (2011) Roles of arbuscular mycorrhizas in plant phosphorus nutrition: interactions between pathways of phosphorus uptake in arbuscular mycorrhizal roots have important implications for understanding and manipulating plant phosphorus acquisition. Plant Physiol 156: 1050–1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SE, Read DJ (2008) Mycorrhizal Symbiosis. Academic Press, Cambridge, UK [Google Scholar]
- Smith SE, Smith FA, Jakobsen I (2004) Functional diversity in arbuscular mycorrhizal (AM) symbioses: the contribution of the mycorrhizal P uptake pathway is not correlated with mycorrhizal responses in growth or total P uptake. New Phytol 162: 511–524 [Google Scholar]
- Stribley DP, Tinker PB, Snellgrove RC (1980) Effeets of vesicular-arbuscular mycorrhizal fungi on the relations of plant growth, internal phosphorus concentration and soil phosphate analyses. J Soil Sci 31: 655–672 [Google Scholar]
- Takeda N, Handa Y, Tsuzuki S, Kojima M, Sakakibara H, Kawaguchi M (2015) Gibberellins interfere with symbiosis signaling and gene expression and alter colonization by arbuscular mycorrhizal fungi in Lotus japonicus. Plant Physiol 167: 545–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tawaraya K, Sasai K, Wagatsuma T (1994) Effect of phosphorus application on the contents of amino acids and reducing sugars in the rhizosphere and VA mycorrhizal infection of white clover. Soil Sci Plant Nutr 40: 539–543 [Google Scholar]
- Thomson BD, Robson AD, Abbott LK (1991) Soil mediated effects of phosphorus supply on the formation of mycorrhizas by Scutellispora calospora (Nicol. and Gerd.) Walker and Sanders on subterranean clover. New Phytol 18: 463–469 [Google Scholar]
- Vance CP. (2001) Symbiotic nitrogen fixation and phosphorus acquisition. Plant nutrition in a world of declining renewable resources. Plant Physiol 127: 390–397 [PMC free article] [PubMed] [Google Scholar]
- Vogel JT, Walter MH, Giavalisco P, Lytovchenko A, Kohlen W, Charnikhova T, Simkin AJ, Goulet C, Strack D, Bouwmeester HJ, Fernie AR, Klee HJ (2010) SlCCD7 controls strigolactone biosynthesis, shoot branching and mycorrhiza-induced apocarotenoid formation in tomato. Plant J 61: 300–311 [DOI] [PubMed] [Google Scholar]
- Volpe V, Giovannetti M, Sun XG, Fiorilli V, Bonfante P (2016) The phosphate transporters LjPT4 and MtPT4 mediate early root responses to phosphate status in non mycorrhizal roots. Plant Cell Environ 39: 660–671 [DOI] [PubMed] [Google Scholar]
- Walder F, Brulé D, Koegel S, Wiemken A, Boller T, Courty PE (2015) Plant phosphorus acquisition in a common mycorrhizal network: regulation of phosphate transporter genes of the Pht1 family in sorghum and flax. New Phytol 205: 1632–1645 [DOI] [PubMed] [Google Scholar]
- Walker N, Smith SE (1984) The quantitative study of mycorrhizal infection, II. The relation of rate of infection and speed of fungal growth to propagule density, the mean length of the infection unit and the limiting value of the fraction of the root infected. New Phytol 96: 55–69 [Google Scholar]
- Wang E, Schornack S, Marsh JF, Gobbato E, Schwessinger B, Eastmond P, Schultze M, Kamoun S, Oldroyd GE (2012) A common signaling process that promotes mycorrhizal and oomycete colonization of plants. Curr Biol 22: 2242–2246 [DOI] [PubMed] [Google Scholar]
- Xue L, Cui H, Buer B, Vijayakumar V, Delaux PM, Junkermann S, Bucher M (2015) Network of GRAS transcription factors involved in the control of arbuscule development in Lotus japonicus. Plant Physiol 167: 854–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SY, Grønlund M, Jakobsen I, Grotemeyer MS, Rentsch D, Miyao A, Hirochika H, Kumar CS, Sundaresan V, Salamin N, et al. (2012) Nonredundant regulation of rice arbuscular mycorrhizal symbiosis by two members of the phosphate transporter1 gene family. Plant Cell 24: 4236–4251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S, Kameoka H, Tempo M, Akiyama K, Umehara M, Yamaguchi S, Hayashi H, Kyozuka J, Shirasu K (2012) The D3 F-box protein is a key component in host strigolactone responses essential for arbuscular mycorrhizal symbiosis. New Phytol 196: 1208–1216 [DOI] [PubMed] [Google Scholar]
- Zhang Q, Blaylock LA, Harrison MJ (2010) Two Medicago truncatula half-ABC transporters are essential for arbuscule development in arbuscular mycorrhizal symbiosis. Plant Cell 22: 1483–1497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Pumplin N, Ivanov S, Harrison MJ (2015) EXO70I is required for development of a sub-domain of the periarbuscular membrane during arbuscular mycorrhizal symbiosis. Curr Biol 25: 2189–2195 [DOI] [PubMed] [Google Scholar]
- van Aarle IM, Olsson PA (2003) Fungal lipid accumulation and development of mycelial structures by two arbuscular mycorrhizal fungi. Appl Environ Microbiol 69: 6762–6767 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.