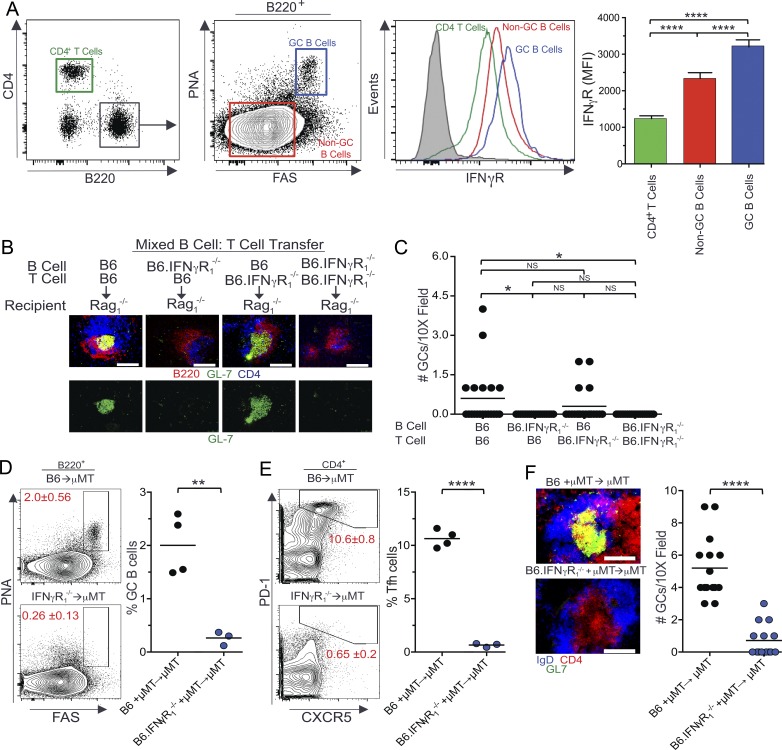
Figure 4.
B cell–intrinsic requirement of IFN-γR signaling in Spt-GC and Tfh cell development. (A) Flow cytometric analysis of surface expression of IFN-γR on CD4+ T cells, B220+PNA−Fas− non-GC B cells, and B220+PNAhiFashi GC B cells. The mean IFN-γR surface expression (mean fluorescence intensity ± SD) in these three cell types from five mice of each genotype is shown in the right panel. Filled gray histogram indicates isotype control. (B) Representative images of spleen sections from Rag1−/− mice (five mice per group) that received combinations of naive B and T cells are shown. Sections were stained with anti-B220, anti-CD4, and GC B cell marker GL-7. Bottom panels show only GC B cell staining with GL-7. (C) Quantification of the number of GCs observed per 10× field from similar spleen sections shown in B (n = 5 mice per group). (D and E) The percentages of B220+FashiPNAhi GC B cells (D) and CD4+CXCR5hiPD-1hi Tfh cells (E) were obtained from flow cytometric analysis of spleen cells of 3-mo-old μMT mice reconstituted with BM cells from B6 and B6.IFN-γR1−/− mice. Each symbol represents a mouse (n = 3-4 mice per group). (F) Representative images of spleen sections from μMT mice (3–4 mice per group) described in D and E are shown (left) in which sections were stained with GL-7, anti-IgD, and anti-CD4. The number of GCs observed per 10× field from these mice (n = 3–4) is shown in the right panel. These data are representative of two independent experiments. In C, statistical analysis was performed by one-way ANOVA with a follow-up Tukey multiple-comparison test. In A, B, and D–F, statistical values were determined using an unpaired, nonparametric, Mann–Whitney Student’s t test. *, P < 0.05; **, P < 0.01; ****, P < 0.001. Bars,150 µm.