Figure 2.
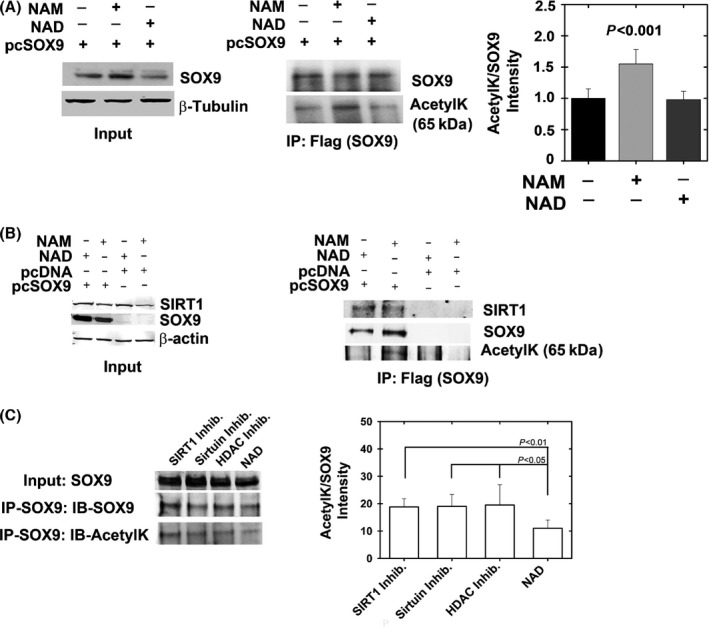
SIRT1 binds and predominantly deacetylates SOX9. HEK293 cells were transfected with a SOX9 expression plasmid (pcSOX9) or pcDNA control and incubated for 24 h with NAD (cofactor of sirtuins) or NAM (a noncompetitive inhibitor of sirtuins). (A) Following immunoblotting for inputs to verify SOX9 expression (right panel), the extracts were immunoprecipitated for flag‐tag and immunoblotted for SOX9 and acetylated lysine (AcetylK), (n = 5). Semi‐quantitative band intensity for acetylated SOX9 is presented in the right graph in A. (B). Left panel shows Flag (SOX9) immunoprecipitants possessing augmented binding to endogenous SIRT1 only when SOX9 was overexpressed. Further, SOX9 protein was not acetylated upon treatment with NAD (sirtuin cofactor) compared with NAM (sirtuin inhibitor) treatment (n = 5). (C) HEK293 cells were transfected with SOX9 expression plasmid and incubated with 1 μm EX‐527 (SIRT1 inhibitor), 10 mm NAM and 50 μm EX‐527 (Sirtuin inhibitors), 10 mm NAM/50 μm EX‐527/1 μm TSA/5 μm Sodium butyrate (HDAC inhibitors), and 10 mm NAD (Sirtuin cofactor). SOX9 was immunoprecipitated and blotted for SOX9 and acetyl‐lysine (n = 7). ImageJ was used for quantification of band intensity (right panel of B) and shows no change in SOX9 acetylation among the inhibitor treatments, confirming that SIRT1 is predominantly responsible for SOX9 deacetylation.
