Figure 5.
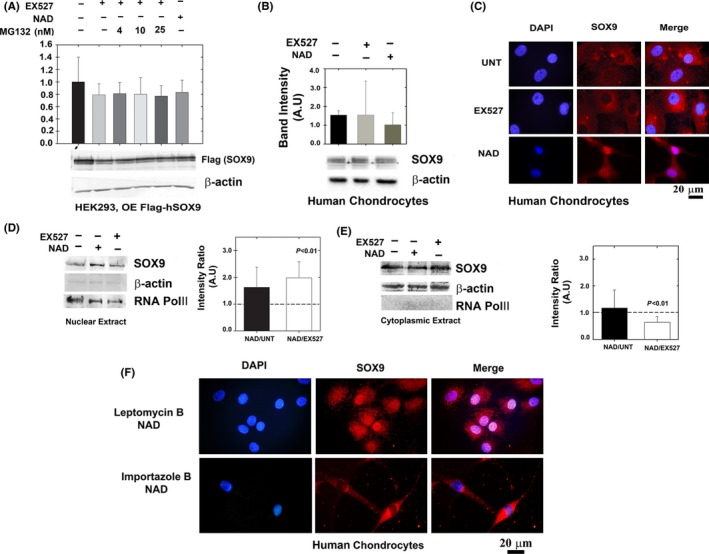
Deacetylated SOX9 does not exhibit enhanced protein stability but possesses enhanced nuclear localization. (A) HEK293 cells were transfected with Flag‐SOX9 expression plasmid (OE; overexpression) and treated with NAD, EX527 and MG132. Levels of SOX9 were monitored by immunoblotting of flag‐tag (n = 5). (B) Cultured human chondrocytes were immunoblotted for SOX9 levels in the presence of EX527 or NAD (n = 5). (C) Confocal microscopy of primary cultured chondrocytes treated as in B (n = 5). Blue florescence for nuclear staining via DAPI; red florescence for staining of endogenously expressed SOX9 via anti‐SOX9 antibody and Alexa‐fluor 568 secondary antibody. Purple florescence indicates overlap of blue and red and implies enhanced SOX9 levels in the nuclear compartment upon NAD treatment. ‘UNT’ denotes untreated cells. The images were magnified x100. (D) Nuclear and (E) cytoplasmic extracts of SOX9 in EX527, NAD and untreated human cultured chondrocytes (n = 5). Semi‐quantitative analysis of RNA POL‐II and β‐actin from nuclear extracts indicates a 9.9% contamination of cytoplasmic proteins, while cytoplasmic extracts possessed a 13% contamination of nuclear proteins, overall indicating that the level of enrichment is significantly high in these extracts. (F) Human chondrocytes cultured on coverslip and treated with 10 nM Leptomycin B and 10 mm NAD or 40 μm Importazole and NAD. Immunostaining and capture of images as indicated in C.
