Figure 6.
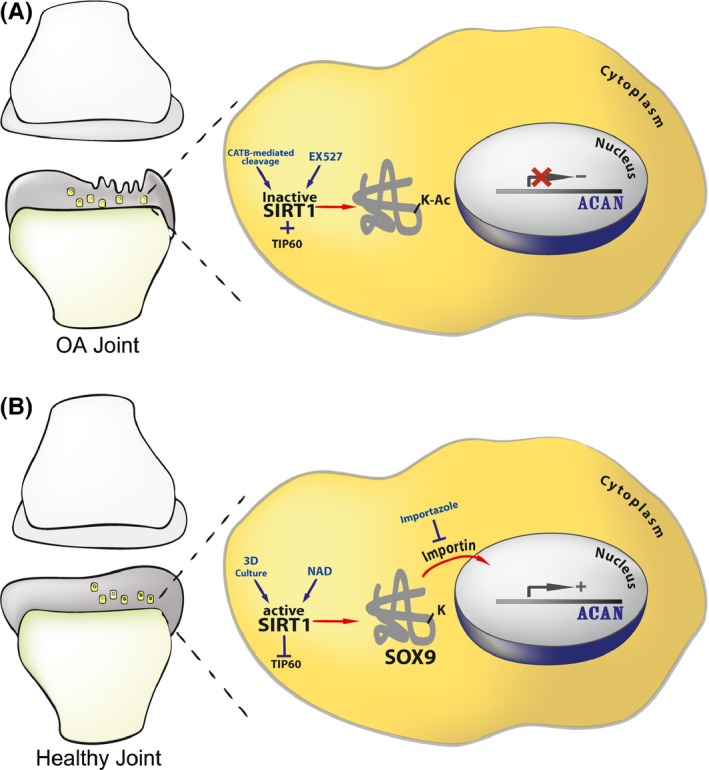
Scheme illustrating the mechanism of SOX9 nuclear entry. The illustration shows how acetylation state could affect SOX9 nuclear entry and ACAN expression in intact and OA cartilage. (A) Proposed mechanism in a degenerating OA joint, wherein cells are less capable of expressing ACAN. This is due to an increase in the acetylation state of SOX9 succeeding SIRT1 inactivation. SIRT1 inactivation is exerted by cathepsin B (CATB)‐mediated cleavage (Dvir‐Ginzberg et al., 2011) or incubation with the SIRT1 inhibitor EX527. In addition to SIRT1 inactivation, TIP60 acetyl‐transferase activity will contribute to SOX9 hyperacetylation (Hattori et al., 2008). (B) Healthy articular cartilage wherein SIRT1 is active. SIRT1 activity is rendered by 3D culture conditions and the presence of SIRT1 activators (i.e. resveratrol, NAD, etc). Enhanced SIRT1 activity will inhibit TIP60 (Wang & Chen, 2010), thereby promoting SOX9 hypo‐acetylated state. Hypo‐acetylated SOX9 is capable of entering the nuclear compartment and binding the enhancer of ACAN to induce gene transactivation and expression. The import of hypo‐acetylated SOX9 to the nuclear compartment is facilitated by importin β, which is specifically inhibited by importazole.
