Abstract
Introduction. The neutrophil-to-lymphocyte ratio (NLR), which can be easily calculated from routine complete blood counts of the peripheral blood, has been suggested to serve as a prognostic factor for some solid malignancies. In the present study, we aimed to determine the relationship between NLR in prostate cancer patients undergoing radical prostatectomy (RP) and their prognosis. Materials and Methods. We assessed NLR in 73 men (patients) who received RP for their prostate cancer. We also performed immunohistochemistry for CD8 and CD66b in a separate set of RP specimens. Results. The median NLR in the 73 patients was 1.85. There were no significant correlations of NLR with tumor grade (p = 0.834), pathological T stage (p = 0.082), lymph node metastasis (p = 0.062), or resection margin status (p = 0.772). Based on the area under the receiver operator characteristic curve (AUROC) to predict biochemical recurrence after RP, potential NLR cut-off point was determined to be 2.88 or 3.88. However, both of these cut-off points did not precisely predict the prognosis. There were no statistically significant differences in the number of CD66b-positive neutrophils or CD8-positive lymphocytes between stromal tissues adjacent to cancer glands and stromal tissues away from cancer glands and between different grades or stages of tumors. Conclusions. There was no association between NLR and biochemical failure after prostatectomy.
1. Introduction
The neutrophil-to-lymphocyte ratio (NLR) can be easily calculated from routine complete blood counts (CBCs) in the peripheral blood. The NLR has been suggested to be not only a predictor of the systemic inflammatory response in critical care patients, but also a prognosticator for some solid malignancies including prostate cancer [1, 2].
Prostate-specific antigen (PSA), also known as human kallikrein 3, has been widely used for the early detection of prostate cancer as well as monitoring of its treatment. However, nonmalignant conditions, in particular benign prostate hyperplasia and acute prostatitis, often raise the serum PSA level, which complicates the diagnosis of prostate cancer using the PSA measurement alone [3, 4]. We previously described the effectiveness of the NLR for predicting prostate cancer in men undergoing prostate biopsy by showing that the NLR was significantly higher in the prostate cancer group compared with the noncancer group [5]. We also confirmed the usefulness of the NLR for predicting the prognosis of prostate cancer patients who presented with metastatic disease and who underwent docetaxel chemotherapy.
In the present study, we aimed to determine whether the NLR served as a prognostic factor in prostate cancer patients undergoing radical prostatectomy (RP).
2. Materials and Methods
2.1. Patients
The NLR was determined in a total of 73 men who subsequently underwent RP at our institution from 2011 to 2014 for their prostate cancer. None of these patients received hormonal therapy or other anticancer treatments preoperatively or postoperatively prior to biochemical recurrence. Biochemical recurrence was defined as a single PSA of ≥0.2 ng/mL. The institutional review board of Yokohama City University Medical Center approved this study.
2.2. Clinical and Laboratory Assessments
The NLR was calculated using the neutrophil and lymphocyte counts via CBCs obtained before RP. To determine the differences in the surgical technique, all RPs were performed or supervised by one expert surgeon (Yasuhide Miyoshi). Tumor grade of the RP specimens was determined according to the ISUP consensus on Gleason grading [6].
2.3. Prostate Tissue Microarray (TMA) and Immunohistochemistry (IHC)
We retrieved separate 51 RP specimens obtained at Yokohama City University Hospital (Yokohama, Japan). Appropriate approval from the institutional review board at our institution was obtained before the construction and use of the TMA. This was constructed from each representative lesion (benign and carcinoma). The clinicopathologic characteristics of these 51 patients are summarized in Table 2.
Table 2.
Clinicopathologic characteristics of prostate cancer patients used for immunohistochemical analysis.
| Number (%) or median (mean ± SD) | |
|---|---|
| Number of patients | 51 |
| Age (years) | 68 (65.1 ± 11.6) |
| Initial PSA (ng/mL) | 12.8 (13.9 ± 7.1) |
| Gleason score | |
| ≦6 | 10 (19.6%) |
| 7 | 28 (54.9%) |
| ≧8 | 13 (25.5%) |
| Pathological T stage | |
| 2 | 30 (58.2%) |
| ≧3 | 20 (39.2%) |
IHC was performed on the sections (5 µm thick) from the prostate TMA, as described previously [7], using a primary antibody to CD66b (clone G10F5, diluted at 1 : 200, BD Biosciences, San Jose, CA, USA) or CD8 (clone C8/144B, diluted at 1 : 100, DAKO Corporation, Carpinteria, CA, USA). The slides were examined by a single pathologist (Hiroshi Miyamoto) blinded to the sample identity. The total numbers of CD66b-positive and CD8-positive cells were counted in each TMA core.
2.4. Statistical Analyses
The patients' characteristics were analyzed using the Mann-Whitney U test, chi-square test, and one-factor ANOVA test. The NLR cut-off value was evaluated by the AUROC curve. The Kaplan-Meier product limit estimator was used to estimate the recurrence-free survival after RP. A log-rank test was used for comparison. All these statistical analyses were performed using the Graph Pad Prism software program (Graph Pad Software, La Jolla, CA, USA). Statistical significance was determined to exist at p < 0.05.
3. Results
3.1. The NLR Values Were Not Correlated with the Pathological Status
A total of 73 patients underwent RP with variable NLRs. The median calculative NLR was 1.85. The clinicopathologic characteristics of these patients are summarized in Table 1.
Table 1.
Patients' characteristics.
| Number (%) or median (mean ± SD) | |
|---|---|
| Age (years) | 66 (66.0 ± 5.95) |
| Initial PSA (ng/mL) | 8.5 (11.0 ± 6.5) |
| NCCN risk criteria | |
| Low | 10 (13.7%) |
| Intermediate | 35 (47.9%) |
| High | 28 (38.4%) |
| NLR | 1.85 (2.20 ± 1.04) |
| Gleason score | |
| ≤6 | 12 (16.4%) |
| 7 | 51 (69.8%) |
| ≥8 | 10 (13.7%) |
| Pathological T stage | |
| 2 | 51 (69.9%) |
| 3 | 21 (28.8%) |
| 4 | 1 (1.4%) |
| Lymph node metastasis | 5 (6.8%) |
| Positive resection margin | 27 (37.0%) |
| PSA failure | 15 (20.5%) |
| Observation period | 20.7 (25.9 ± 20.8) |
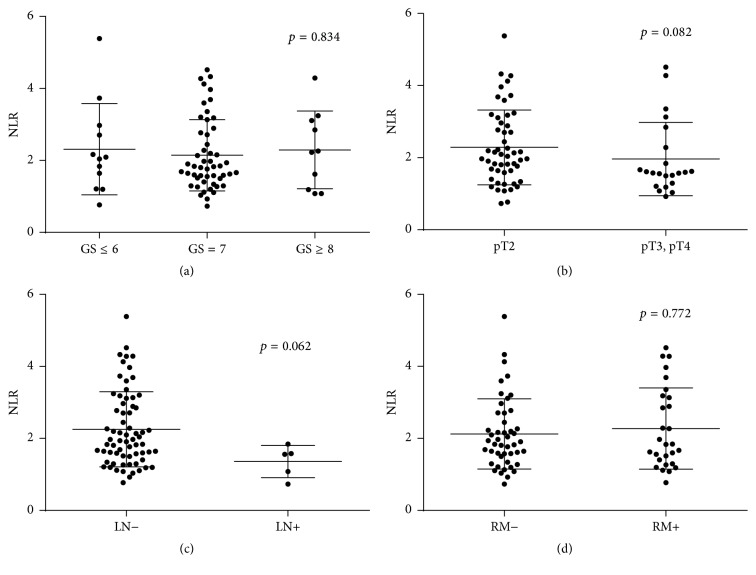
The NLR was not significantly correlated with histopathological features, including Gleason score (GS) (≤6 versus 7 versus ≥8, p = 0.834), pathological T stage (pT2 versus ≥pT3, p = 0.082), lymph node metastasis (negative versus positive, p = 0.062), or surgical margin status (negative versus positive, p = 0.772) (Figure 1).
Figure 1.
Comparison of the NLR with each prognostic factor, including (a) Gleason score, (b) pathological T stage, (c) lymph node metastasis, or (d) surgical margin status.
3.2. The NLR Values Were Not Correlated with PSA Failure
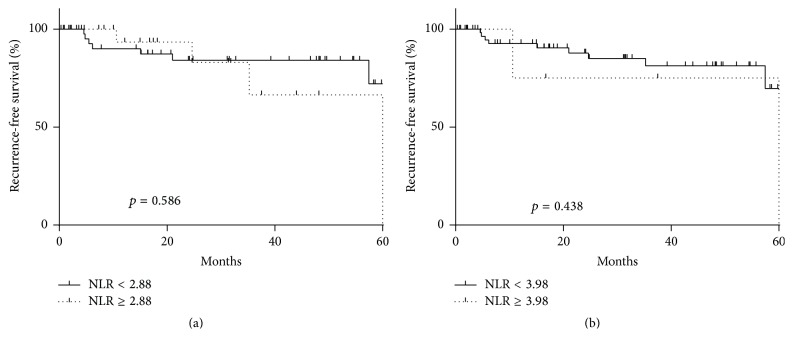
Based on the AUROC curve, potential NLR cut-off point was 2.88 or 3.88 to predict PSA failure (AUC: 0.5092). The patients include 55 in low NLR group and 18 in high NLR group (NLR cut-off: 2.88). And median PSA recurrence-free survival was 63.8 months. However, neither of these cut-off points precisely predicted PSA recurrence after RP (Figure 2).
Figure 2.
The recurrence-free survival according to the NLR.
3.3. Infiltrating Neutrophils and Lymphocytes in Prostate Cancer Specimens
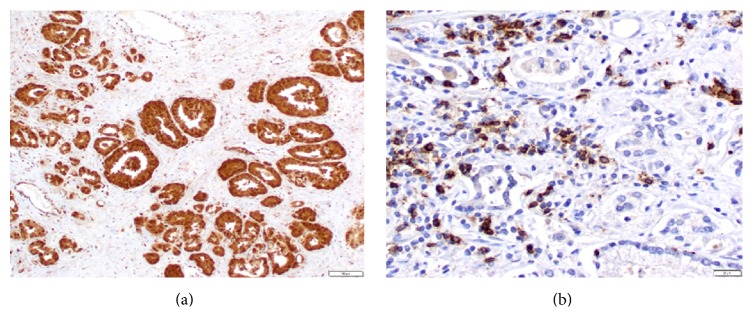
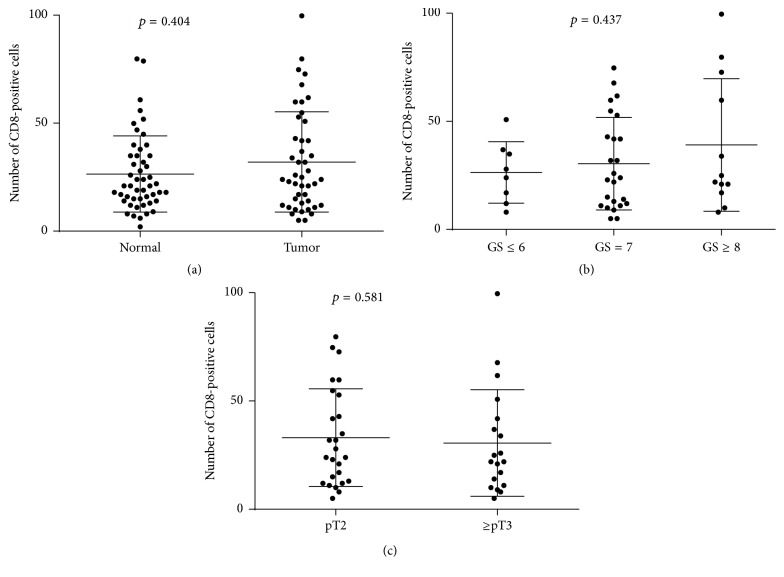
Infiltrating CD66b-positive cells in the stroma were observed only in a few cases, while tumor cells were immunoreactive for CD66b in several cases (Figure 3(a)). Therefore, we analyzed the relationship between the number of infiltrating CD8-positive lymphocytes (Figure 3(b)) and clinicopathological features of prostate cancer. The number of CD8-positive cells in the stroma adjacent to the tumors was not significantly higher than that in the stroma around nonneoplastic prostate (p = 0.404; Figure 4(a)). In addition, there were no significant correlations between the number of CD8-positive lymphocytes and tumor grade (p = 0.437; Figure 4(b)) or pathological T stage (p = 0.581; Figure 4(c)).
Figure 3.
Immunohistochemical staining for (a) C66b and (b) CD8.
Figure 4.
Number of CD8-positive cells in (a) normal and tumor tissues, (b) different GS, and (c) different pathological T stage.
4. Discussion
There is increasing evidence correlating the presence of systemic inflammation with a poorer cancer-specific survival in patients with several solid tumors, such as colorectal carcinoma [8–14]. Moreover, nonsteroidal anti-inflammatory medications have been suggested to reduce the risk of developing prostate cancer, implying a critical correlation between inflammation and prostate carcinogenesis [8, 9]. It has previously been demonstrated that the presence of an inflammatory response can be determined by both the expression of C-reactive protein and/or an elevation in the NLR [10, 15, 16]. In particular, the latter has been associated with a poorer prognosis in patients with prostate cancer [17].
Biochemical recurrence after RP has been associated with multiple factors, including the preoperative PSA level, the pathological stage, the GS of RP specimen, and the surgical margin status [18, 19]. Although our study confirmed these observations, we did not find strong associations between the NLR and any prognostic or clinicopathological factors. Regarding the NLR for the patients who received RP, some reports showed the effectiveness of the NLR as a predictor of biochemical recurrence [17, 20–22]. On the other hand, for the patients who have low-risk prostate cancer, the NLR was not a useful predictor for biochemical recurrence [23].
IHC was performed to detect CD66b-positive neutrophils and CD8-positive lymphocytes in RP specimens. However, there was no significant difference in the number of infiltrating CD66b-positive or CD8-positive cells between tissues from normal-appearing prostate and prostate cancer. Furthermore, no significant correlations between the neutrophil number, lymphocyte number, or their ratio and tumor characteristics (e.g., GS and pathological stage) or patient outcome were observed. A previous immunohistochemical study in esophageal squamous cell carcinoma specimens demonstrated that intratumoral neutrophils, CD8-positive lymphocytes, and their ratio, as seen in the NLR in CBCs, correlated with disease progression [24]. However, no attempts in other tissue specimens have been made to determine the role of the NLR in tumorigenesis or tumor progression as a biomarker.
Cho et al. reported that patients with an elevated NLR exhibit a relative lymphocyte-mediated immune response to malignancy, thereby worsening their prognosis and increasing the potential for tumor progression [25, 26]. The interaction between the tumor and host immune system promotes tumor cell proliferation, metastasis, and activation of the inflammatory cascade in the host, which further deteriorates the general condition of cancer patients [27].
There are several limitations associated with this study. First, the study was retrospective in nature and had a limited sample size, as we enrolled patients who obtained CBCs preoperatively from 73 constitutive patients in our institution. On the other hand, the pathological results of RP were often dependent on the surgical procedure; thus, to exclude this bias we obtained the data only from the cases that underwent RP by one surgeon (Yasuhide Miyoshi). Additionally, the sample size of this study was small compared with our previous study of prostate needle biopsies [13]. The NLR as a prognostic factor in solid malignancies was previously reported mainly in patients with advanced stages [17, 28, 29]. Because the patients who received RP had localized prostate cancer, the differences may be smaller. Finally, an immunohistochemical analysis of CD8 and CD66b was performed using another patient cohort.
In conclusion, in the current study, there was no association between NLR and biochemical failure after prostatectomy. Further investigation is needed to confirm our results.
Acknowledgments
The authors would like to thank R. Shimizu for assistance with immunohistochemical staining. Grants from the Uehara Memorial Foundation, the Tokyo Biochemical Research Foundation, the Japanese Foundation for Research and Promotion of Endoscopy, and the Yokohama Foundation for Advancement of Medical Science and an International Exchange Grant from Kato Memorial Bioscience Foundation were provided to Takashi Kawahara No grant numbers were applied.
Competing Interests
The authors declare that they have no competing interests.
Authors' Contributions
Takashi Kawahara and Yoko Maeda contributed equally to this work.
References
- 1.Gu X., Gao X., Li X., et al. Prognostic significance of neutrophil-to-lymphocyte ratio in prostate cancer: evidence from 16,266 patients. Scientific Reports. 2016;6 doi: 10.1038/srep22089.22089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yin X., Xiao Y., Li F., Qi S., Yin Z., Gao J. Prognostic role of neutrophil-to-lymphocyte ratio in prostate cancer: a systematic review and meta-analysis. Medicine. 2016;95(3) doi: 10.1097/md.0000000000002544.e2544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Polascik T. J., Oesterling J. E., Partin A. W. Prostate specific antigen: a decade of discovery—what we have learned and where we are going. The Journal of Urology. 1999;162(2):293–306. doi: 10.1016/s0022-5347(05)68543-6. [DOI] [PubMed] [Google Scholar]
- 4.Erol B., Gulpinar M. T., Bozdogan G., et al. The cutoff level of free/total prostate specific antigen (f/t PSA) ratios in the diagnosis of prostate cancer: a validation study on a Turkish patient population in different age categories. The Kaohsiung Journal of Medical Sciences. 2014;30(11):545–550. doi: 10.1016/j.kjms.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 5.Kawahara T., Fukui S., Sakamaki K., et al. Neutrophil-to-lymphocyte ratio predicts prostatic carcinoma in men undergoing needle biopsy. Oncotarget. 2015;6(31):32169–32176. doi: 10.18632/oncotarget.5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Epstein J. I., Allsbrook W. C., Jr., Amin M. B., et al. The 2005 International Society of Urological Pathology (ISUP) consensus conference on Gleason grading of prostatic carcinoma. The American Journal of Surgical Pathology. 2005;29(9):1228–1242. doi: 10.1097/01.pas.0000173646.99337.b1. [DOI] [PubMed] [Google Scholar]
- 7.Kawahara T., Shareef H. K., Aljarah A. K., et al. ELK1 is up-regulated by androgen in bladder cancer cells and promotes tumor progression. Oncotarget. 2015;6(30):29860–29876. doi: 10.18632/oncotarget.5007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kawahara T., Ishiguro H., Hoshino K., et al. Analysis of NSAID-activated gene 1 expression in prostate cancer. Urologia Internationalis. 2010;84(2):198–202. doi: 10.1159/000277599. [DOI] [PubMed] [Google Scholar]
- 9.Ishiguro H., Kawahara T. Nonsteroidal anti-inflammatory drugs and prostatic diseases. BioMed Research International. 2014;2014:6. doi: 10.1155/2014/436123.436123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gomez D., Morris-Stiff G., Toogood G. J., Lodge J. P. A., Prasad K. R. Impact of systemic inflammation on outcome following resection for intrahepatic cholangiocarcinoma. Journal of Surgical Oncology. 2008;97(6):513–518. doi: 10.1002/jso.21001. [DOI] [PubMed] [Google Scholar]
- 11.Coussens L. M., Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gunter M. J., Stolzenberg-Solomon R., Cross A. J., et al. A prospective study of serum C-reactive protein and colorectal cancer risk in men. Cancer Research. 2006;66(4):2483–2487. doi: 10.1158/0008-5472.CAN-05-3631. [DOI] [PubMed] [Google Scholar]
- 13.Zhang K., Kaufman R. J. From endoplasmic-reticulum stress to the inflammatory response. Nature. 2008;454(7203):455–462. doi: 10.1038/nature07203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawahara T., Ito H., Terao H., et al. Ureteroscopy assisted retrograde nephrostomy: a new technique for percutaneous nephrolithotomy (PCNL) BJU International. 2012;110(4):588–590. doi: 10.1111/j.1464-410x.2011.10795.x. [DOI] [PubMed] [Google Scholar]
- 15.Zahorec R. Ratio of neutrophil to lymphocyte counts—rapid and simple parameter of systemic inflammation and stress in critically ill. Bratislavske Lekarske Listy. 2001;102(1):5–14. [PubMed] [Google Scholar]
- 16.McMillan D. C., Canna K., McArdle C. S. Systemic inflammatory response predicts survival following curative resection of colorectal cancer. The British Journal of Surgery. 2003;90(2):215–219. doi: 10.1002/bjs.4038. [DOI] [PubMed] [Google Scholar]
- 17.Minardi D., Scartozzi M., Montesi L., et al. Neutrophil-to-lymphocyte ratio may be associated with the outcome in patients with prostate cancer. SpringerPlus. 2015;4, article 255 doi: 10.1186/s40064-015-1036-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Servoll E., Vlatkovic L., Sæter T., et al. The length of a positive surgical margin is of prognostic significance in patients with clinically localized prostate cancer treated with radical prostatectomy. Urologia Internationalis. 2014;93(3):289–295. doi: 10.1159/000362342. [DOI] [PubMed] [Google Scholar]
- 19.Wright J. L., Dalkin B. L., True L. D., et al. Positive surgical margins at radical prostatectomy predict prostate cancer specific mortality. The Journal of Urology. 2010;183(6):2213–2218. doi: 10.1016/j.juro.2010.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang G.-M., Zhu Y., Ma X.-C., et al. Pretreatment neutrophil-to-lymphocyte ratio: a predictor of advanced prostate cancer and biochemical recurrence in patients receiving radical prostatectomy. Medicine. 2015;94(41) doi: 10.1097/md.0000000000001473.e1473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee H., Jeong S. J., Hong S. K., Byun S.-S., Lee S. E., Oh J. J. High preoperative neutrophil–lymphocyte ratio predicts biochemical recurrence in patients with localized prostate cancer after radical prostatectomy. World Journal of Urology. 2015 doi: 10.1007/s00345-015-1701-6. [DOI] [PubMed] [Google Scholar]
- 22.Gazel E., Tastemur S., Acikgoz O., et al. Importance of neutrophil/lymphocyte ratio in prediction of PSA recurrence after radical prostatectomy. Asian Pacific Journal of Cancer Prevention. 2015;16(5):1813–1816. doi: 10.7314/APJCP.2015.16.5.1813. [DOI] [PubMed] [Google Scholar]
- 23.Kwon Y. S., Han C. S., Yu J. W., et al. Neutrophil and lymphocyte counts as clinical markers for stratifying low-risk prostate cancer. Clinical Genitourinary Cancer. 2016;14(1):e1–e8. doi: 10.1016/j.clgc.2015.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McDonald A. C., Vira M. A., Vidal A. C., Gan W., Freedland S. J., Taioli E. Association between systemic inflammatory markers and serum prostate-specific antigen in men without prostatic disease—the 2001–2008 National Health and Nutrition examination survey. The Prostate. 2014;74(5):561–567. doi: 10.1002/pros.22782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gondo T., Nakashima J., Ohno Y., et al. Prognostic value of neutrophil-to-lymphocyte ratio and establishment of novel preoperative risk stratification model in bladder cancer patients treated with radical cystectomy. Urology. 2012;79(5):1085–1091. doi: 10.1016/j.urology.2011.11.070. [DOI] [PubMed] [Google Scholar]
- 26.Cho H., Hur H. W., Kim S. W., et al. Pre-treatment neutrophil to lymphocyte ratio is elevated in epithelial ovarian cancer and predicts survival after treatment. Cancer Immunology, Immunotherapy. 2009;58(1):15–23. doi: 10.1007/s00262-008-0516-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mantovani A., Allavena P., Sica A., Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 28.van Soest R. J., Templeton A. J., Vera-Badillo F. E., et al. Neutrophil-to-lymphocyte ratio as a prognostic biomarker for men with metastatic castration-resistant prostate cancer receiving first-line chemotherapy: data from two randomized phase III trials. Annals of Oncology. 2015;26(4):743–749. doi: 10.1093/annonc/mdu569. [DOI] [PubMed] [Google Scholar]
- 29.Sonpavde G., Pond G. R., Armstrong A. J., et al. Prognostic impact of the neutrophil-to-lymphocyte ratio in men with metastatic castration-resistant prostate cancer. Clinical Genitourinary Cancer. 2014;12(5):317–324. doi: 10.1016/j.clgc.2014.03.005. [DOI] [PubMed] [Google Scholar]