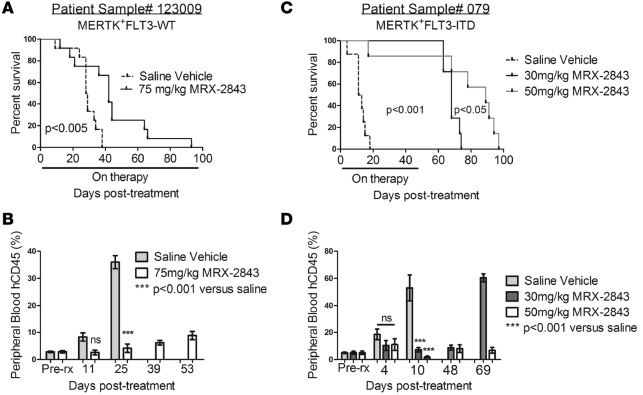
Figure 5. MRX-2843 prolongs survival in patient-derived xenograft models of AML.
(A and C) Kaplan-Meier curves showing survival of NSGS mice xenografted with the indicated patient sample and treated with MRX-2843 or vehicle (saline). (B and D) Peripheral disease burden (percentage of hCD45+ peripheral blood mononuclear cells) was determined by flow cytometry 3 days prior to initiation of treatment (Pre-rx) and on the indicated days after treatment. Mean values and standard errors are shown. (A and B) Mice were started on therapy 53 days after transplantation with leukemia cells (day 0). Median survival of MRX-2843–treated mice after treatment was 42 days compared with 28.5 days for vehicle-treated controls (n = 12 per group). P < 0.005, by log-rank test (A); ***P < 0.001 versus saline, by 1-way ANOVA (B). (C and D) Mice were started on therapy 42 days after transplantation (day 0). Median survival of MRX-2843–treated mice was 68 days (30 mg/kg MRX-2843) and 89 days (50 mg/kg MRX-2843) after treatment compared with 12 days for vehicle-treated controls (n ≥8 per group). P < 0.001 and P < 0.05, by log-rank test (C); ***P < 0.001 versus saline, by 1-way ANOVA (D). AML, acute myeloid leukemia; FLT3, FMS-like tyrosine kinase 3; hCD45+, human CD45+; NSGS, NOD-SCID-γ mice expressing Tg human cytokines. NOD-SCID-γ mice expressing Tg human cytokines