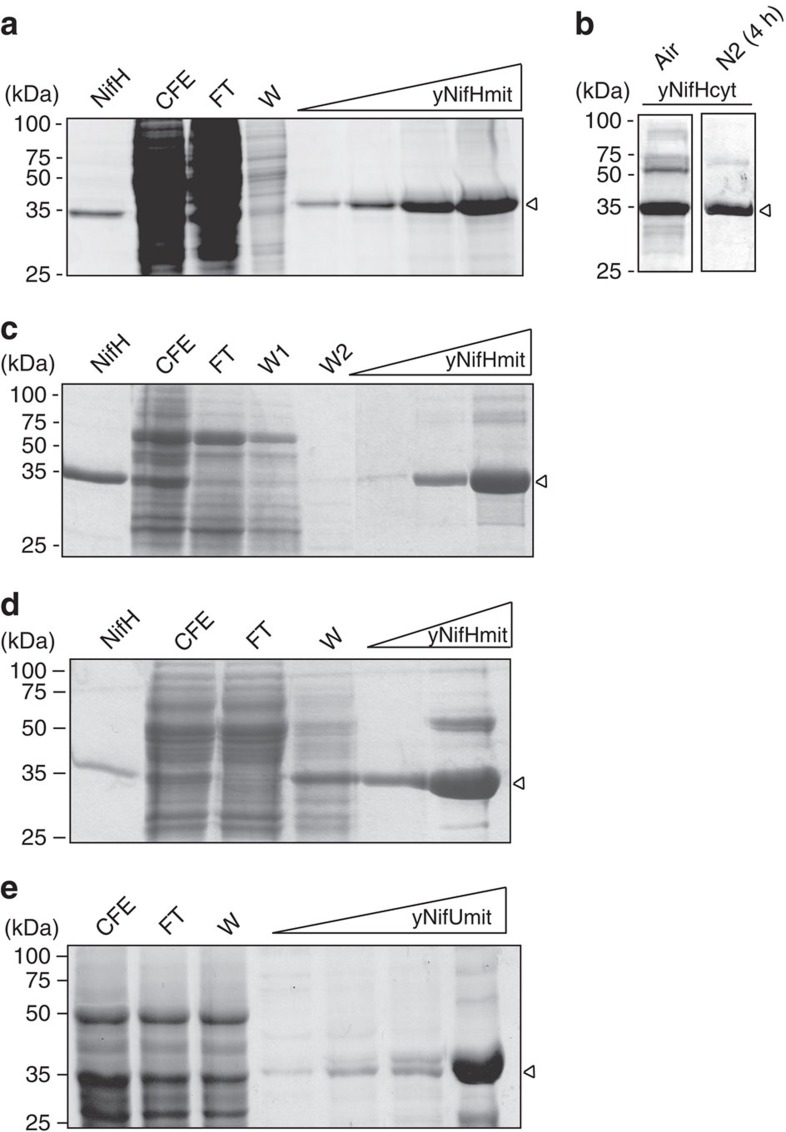
Figure 2. Purification of yNifH and yNifU proteins.
Aerated cultures (1 litre air·per minute·per litre of culture) of galactose-induced S. cerevisiae cells were used as source of Nif proteins. Co2+or Ni2+ affinity columns were used for the purification of his-tagged yNifH and yNifU, respectively. The migration of molecular weight markers in SDS–PAGE is indicated at the left side of each panel. Purified A. vinelandii NifH was added as control in a, c and d. CFE, cell-free extracts; FT, flow-through fractions; W, protein fractions eluted after washing with washing buffer; yNifHmit, yNifHcyt and yNifUmit indicate protein fractions eluted after applying imidazole to the affinity chromatography columns. Arrows point to purified NifH and NifU proteins. (a) yNifHmit purification from GF2 cells co-expressing mitochondria-targeted NifH and NifM. (b) yNifHcyt purified from aerated GF9 cells co-expressing cytosol-targeted NifH and NifM that were collected under aerobic conditions (air) or subjected to 4 h of intense N2 sparging before cell collection (N2). (c) yNifHmit purification from GF12 cells, expressing mitochondria-targeted NifH in the absence of NifM. (d) yNifHmit purification from GF11 cells co-expressing mitochondria-targeted tagged NifH and NifM along with non-tagged NifU and NifS. (e) yNifUmit purification from GF6 cells co-expressing mitochondria-targeted NifU and NifS.