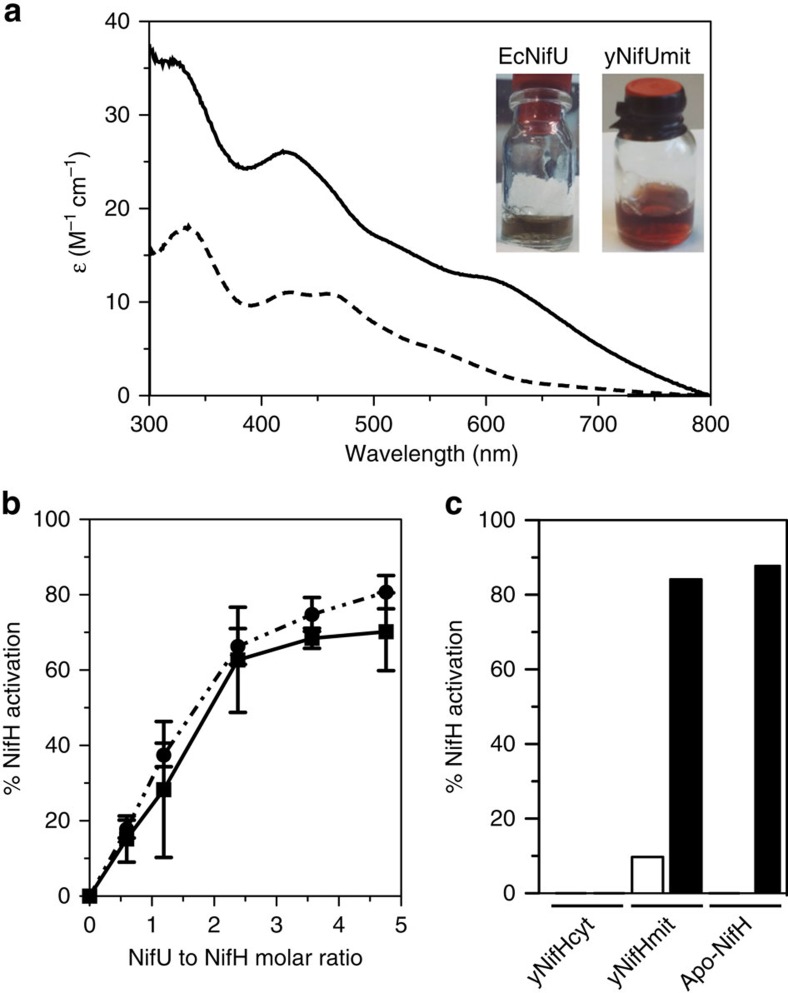
Figure 3. Characterization of purified yNifUmit.
(a) Purified preparations and ultraviolet–visible spectra of yNifUmit (190 μM, broken line) and EcNifU (190 μM, solid line). (b) Titration of apo-NifH activation by yNifUmit (broken line; n=4) or EcNifU (solid line; n=2). Data represent means±s.d. All assays contained 5 μM apo-NifH (162 μg) in the Fe-S cluster reconstitution phase. Fully active Fe protein supported MoFe protein specific activity of 2,200 nmol ethylene·per minute·per milligram MoFe protein (corresponding to 100% activity). (c) Activation assays of yNifHmit (40 μg) and yNifHcyt (80 μg) with a 5-fold molar excess of yNifUmit. The yNifHmit and yNifHcyt proteins were purified from aerated cultures (1 litre air·per minute per litre of culture) of GF2 and GF9 strains, respectively. In vitro generated A. vinelandii apo-NifH (80 μg) was used as control of activation reactions. MoFe protein-specific activity supported by fully active Fe protein under same assay conditions was 1,480 nmol ethylene· per minute per milligram MoFe protein (corresponding to 100% activity). Empty bar represents the Fe protein activity of purified yNifHmit before the yNifUmit-dependent activation assay. Non-activated A. vinelandii apo-NifH and yNifHcyt lacked Fe protein activity. yNifUmit was unable to endow yNifHcyt with Fe protein activity.