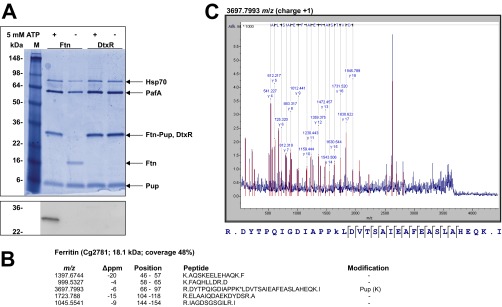
Fig. S5.
In vitro pupylation of the iron storage protein Ftn. (A) Ftn (2 µM) was incubated for 16 h at room temperature with Pup (10 µM) and the Pup ligase PafA (0.5 µM) in the presence or absence of 5 mM ATP. As a negative control, the transcriptional regulator DtxR with a C-terminal His-tag (2 µM) was used. Aliquots of the samples were subjected to SDS/PAGE and Coomassie staining (Upper) or subsequent immunoblotting using anti-Pup antiserum (1:2,000; Lower). Hsp70 is an E. coli chaperone that coeluted with PafA during purification. (B) Result of the peptide mass fingerprint analysis of trypsinized ferritin obtained after in vitro pupylation. Five peptides were detected (48% sequence coverage), including the one covering amino acid residues 66–97 which contains K78 found to be pupylated in our previous pupylome study (23). The predicted m/z (i.e., monoisotopic mass) of the nonpupylated peptide is 3,454.7382, and the measured m/z value was 3,697.7993, corresponding to a difference of 243.0611 (expected for pupylation: 243.0855). (C) The identity of the peptide with m/z of 3,697.7993 was confirmed by MS/MS analysis by the indicated y-ion series.