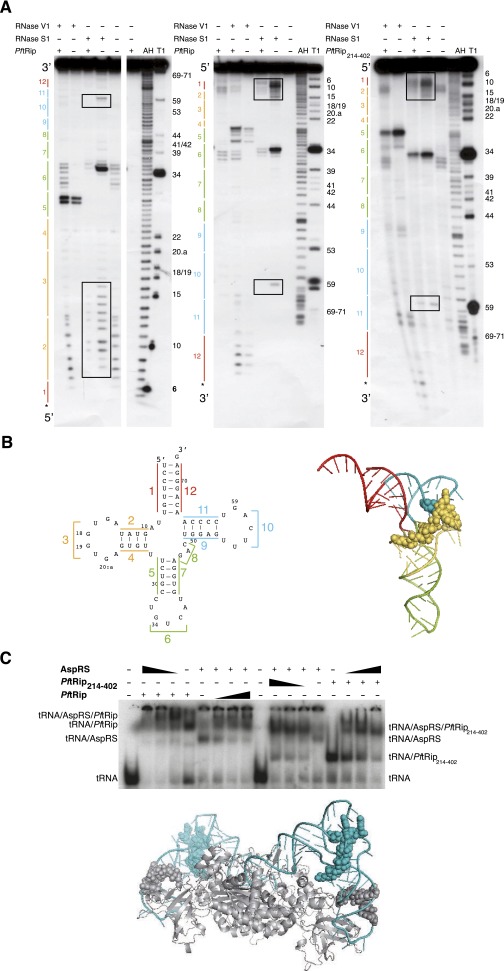
Fig. S3.
PftRip footprint on yeast tRNAAsp. (A) Autoradiographs of RNase V1 (cuts double-stranded and structured RNA regions) and RNase S1 (cuts single-stranded RNA regions) cleavage patterns of tRNAAsp in the presence (+) or in the absence (−) of PftRip or PftRip214–402. The tRNAAsp molecule was [32P] labeled at its 5′ (Left) or 3′ (Center and Right) end. The tRNA architecture described in B is schematized on the left side and nucleotide positions are indicated on the right side of the autoradiographs. Controls without RNases (with or without the PftRip recombinant proteins), as well as alkaline hydrolysis (AH) and RNase T1 (T1) ladders, were run simultaneously. Protections were only considered when present in all three experiments. The tRNA anticodon domain (regions 5, 6, and 7) was somewhat protected from S1 and simultaneously became accessible to V1 in the presence of PftRip214–402, suggesting long-range structural changes in the tRNA on binding. However, these reactivity changes toward S1 and V1 were intensified with PftRip, indicating that the N-terminal domain of tRip caused additional steric hindrance. (B) Secondary (Left) and tertiary (Right) structures of tRNAAsp. Each tRNA domain is indicated in a different color: in red (acceptor arm formed by strands 1 and 12), yellow (d-branch, formed by strands 2, 3, and 4), green (anticodon/variable region-branch, strands 5, 6, 7, and 8), and blue (T-branch, strands 9, 10, and 11). Protections of nucleotides within the D and T branches against RNases (squared in A) are shown with yellow and blue spheres, respectively. (C) Simultaneous binding of yeast aspartyl-tRNA synthetase (AspRS) and PftRip214–402 on the same tRNAAsp molecule and model of their potential interaction. tRNAAsp was incubated with either PftRip and AspRS (Left) or PftRip214–402 and AspRS (Right). The interactions based on footprinting experiments were modeled on the crystal structure of the yeast tRNAAsp (blue)/AspRS (gray) complex (13). Gray spheres indicate the protection of tRNAAsp by the AspRS N-terminal extension (38) and blue spheres correspond to PftRip footprinting.