Figure 1.
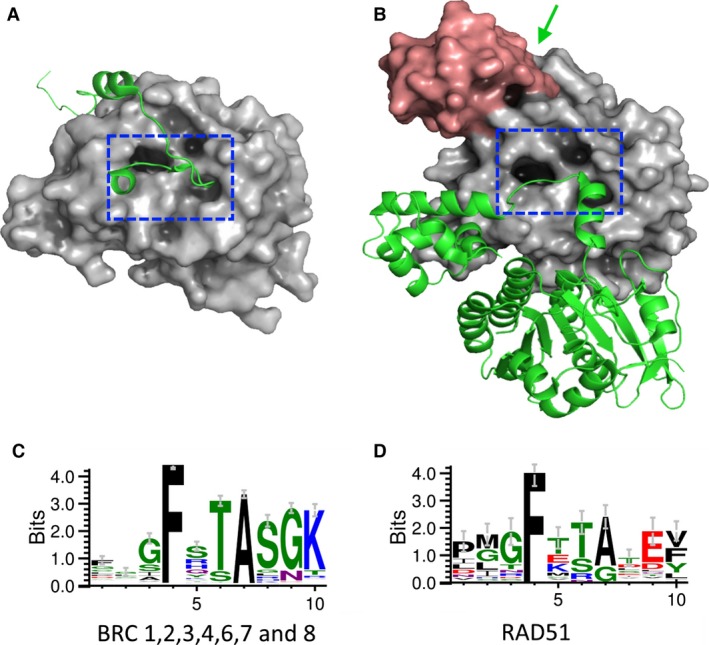
Conservation of FxxA motif (A) BRC4 peptide (green cartoon) bound to truncated human RAD51 (grey surface) (PDB: 1n0w, 11). The blue dashed box highlights the FxxA interaction pocket. (B) Two interacting protein molecules of RAD51 from Saccharomyces cerevisiae are shown. One RAD51 (green cartoon) interacts with another molecule of RAD51 (grey and pink surface) via the FxxA pocket indicated by the dashed blue box. The N‐terminal domain of one RAD51 protomer is highlighted in pink for clarity and the green arrow indicates the location of this protomer's FxxA oligomerisation sequence (PDB: 1szp, 29). (C) Conservation of FxxA motif across the human BRC repeats and (D) across 21 eukaryotic RAD51s and 24 RadAs, with the size of the letters proportional to the degree of conservation. Sequence figures generated using Weblogo 3.0 21, sequence details are found in the Supporting Information.
