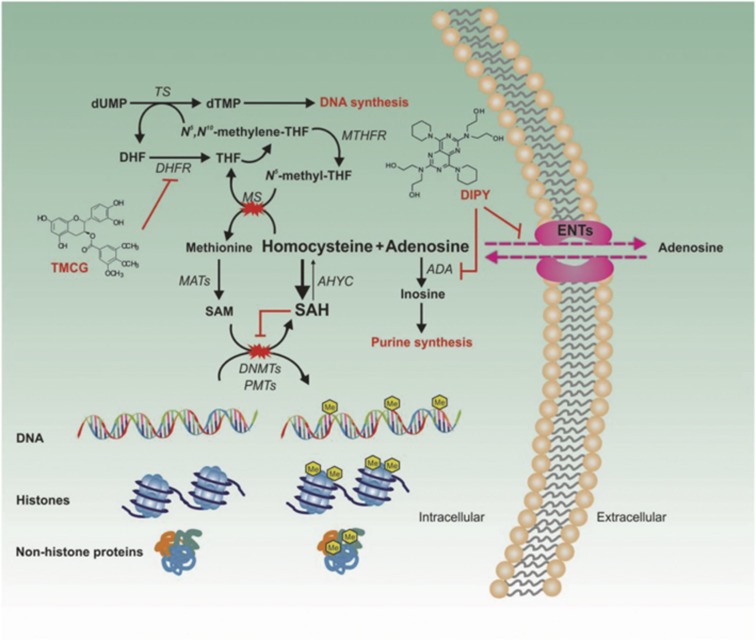
Figure 1.
The strategy used to target the epigenetic machinery of BC cells. Experimental results, accumulated during the last decade, have suggested that the methionine cycle may be a valuable therapeutic target.40, 41 In a recent study, we observed that a combined therapy aimed at uncoupling adenosine metabolism using dipyridamole (DIPY) in the presence of a new synthetic antifolate (TMCG) simultaneously and efficiently blocked both the folic and methionine cycles in BC cells, resulting in massive cell death.12 The TMCG/DIPY combination acted as a HMT that reactivated RASSF1A expression and induced E2F1-mediated apoptosis in BC cells. The schematic illustrates the methionine cycle and its connections with several metabolic and survival cell pathways. Adenosine is efficiently metabolized by specific enzymes (such as ADA and adenosine kinase) before its use in purine nucleotide synthesis, which is particularly important for DNA synthesis in highly proliferating cells. Excess adenosine can be transported out of the cells by ENTs, which are bidirectional transporters that allow adenosine release and uptake by facilitating diffusion along its concentration gradient. However, in the presence of an antifolate compound, adenosine accumulation may become a severe problem for the cell. In folate-deficient cells, the resulting accumulation of homocysteine drives AHYC to catalyze the energetically favorable reverse reaction and to synthesize SAH, a potent inhibitor of cellular methyltransferases. ADA, adenosine deaminase; AHYC, S-adenosylhomocysteine hydrolase; DHF, dihydrofolate; DHFR, dihydrofolate reductase; DNMT, DNA methyltransferase; dTMP, deoxythymidine 5′-monophosphate; dUMP, deoxyuridine monophosphate; ENT, equilibrative nucleoside transporter; MAT, methionine adenosyltransferase; MS, methionine synthase; MTHFR, 5,10-methylenetetrahydrofolate reductase; PMT, protein methyltransferase; SAH, S-adenosylhomocysteine; SAM, S-adenosylmethionine; THF, tetrahydrofolate; TS, thymine synthase