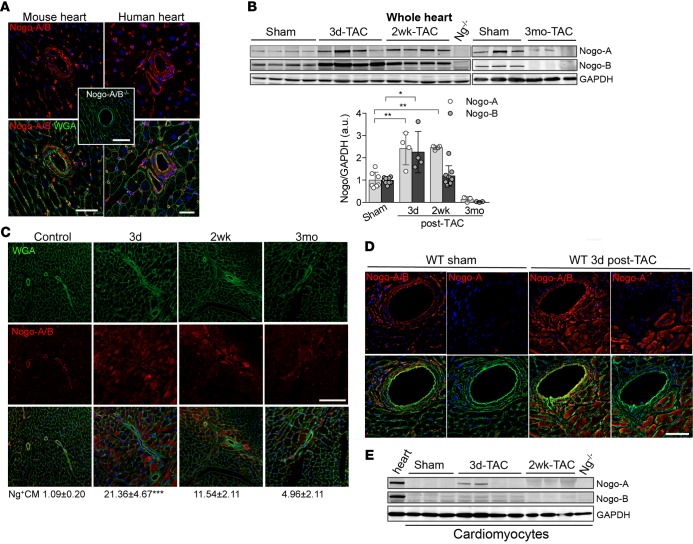
Figure 1. Transient and cell-type–specific overexpression of Nogo-A and Nogo-B in banded hearts.
(A) Immunofluorescence staining of Nogo-A/B (red) and membrane glycoproteins with wheat germ agglutinin (WGA; green) in murine and human hearts. The inset shows the staining for Nogo-A/B in Nogo-A/B–deficient heart sections, as a negative control. The nuclei were counterstained with DAPI (blue). Scale bar: 50 μm. (B) Western blot analysis and quantification of Nogo-A and Nogo-B expression in lysates of hearts of transverse aortic constriction–operated (TAC-operated) and sham-operated WT mice at indicated time points. Nogo-A/B–deficient hearts (Ng–/–) were used as a negative control. GAPDH was used as loading control. (C) Sections of hearts from sham- and TAC-operated WT mice at indicated time points were stained for Nogo-A/B (red), WGA (green), and DAPI (blue). Cardiomyocytes positive for Nogo-A/B were counted in sections from the base, center, and apex of the hearts and are expressed as a percentage of the total number of cardiomyocytes counted per heart section, as shown below images. n = 5/group. Scale bar: 100 μm. (D) Serial sections of sham-operated hearts and hearts 3 days after TAC were stained with anti–Nogo-A antibody and anti–Nogo-A/B antibodies. Nogo-A staining localized exclusively in cardiomyocytes and was not observed in the vasculature or fibroblasts. Scale bar: 50 μm. (E) Western blot analysis of Nogo-A and Nogo-B in cardiomyocytes isolated from sham- and TAC-operated WT hearts at indicated time points. Lysates prepared from the whole WT hearts were used as positive control, whereas lysate from Nogo-A/B–deficient heart was used as negative control. GAPDH was used as loading control. Data are expressed as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001 (B and C), based on 1-way ANOVA followed by Tukey’s multiple comparison test.