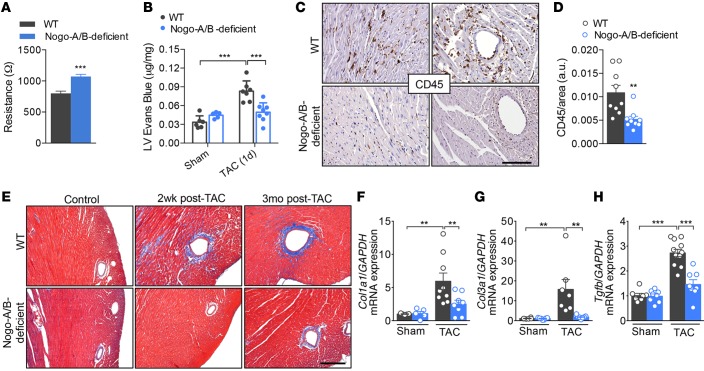
Figure 4. Nogo-A/B–deficient mice were resistant to myocardial permeability, inflammation, and fibrosis.
(A) Assessment of the endothelial barrier functions by measuring the resistance (Ω) of primary cultures of WT and Nogo-A/B–deficient endothelial cells using the Electric Cell-Substrate Impedance Sensing System. n = 14 replicates from 4 independent endothelial cell isolations/group. (B) Myocardial permeability was assessed by quantifying the extravasation of Evans Blue dye into the hearts of sham- or transverse aortic constriction–operated (TAC-operated) WT and Nogo-A/B–deficient mice and was expressed as μg/mg dry heart weight. n ≥ 6/group. (C) Images and (D) quantification of immunohistochemical staining of CD45 in heart sections from WT and Nogo-A/B–deficient mice at 3 days after TAC. Scale bar: 100 μm. n ≥ 9/group. (E) Masson’s trichrome staining of cardiac fibrosis in TAC- or sham-operated WT and Nogo-A/B–deficient mice at indicated time points. Scale bar: 200 μm. Real-time PCR analysis of expression of fibrosis-related genes (F) collagen type I α 1 (Col1a1), (G) collagen type 3 α 1 (Col3a1), and (H) Tgfb in sham or 2 weeks banded WT and Nogo-A/B–deficient hearts. Gapdh served as internal control. Data were normalized to sham group. n ≥ 9/group. Data are expressed as mean ± SEM. **P < 0.01, ***P < 0.001 compared with WT or as otherwise indicated. Statistical significance was determined by unpaired t test (A and D) or 1-way ANOVA followed by Tukey’s multiple comparison test (B and F–H).