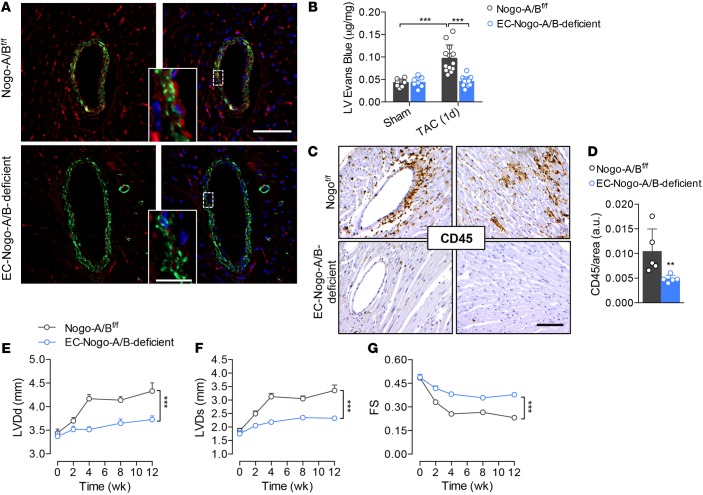
Figure 5. Endothelial Nogo-B controls the progression of maladaptive left ventricle remodeling and systolic dysfunction induced by pressure overload.
(A) Immunofluorescence staining of Nogo-A/B (red) and α-smooth muscle actin (α-SMA; green) in sections of heart tissue from Nogo-A/Bf/f and EC-Nogo-A/B–deficient mice. Scale bar: 50 μm. Inset, higher-magnification view of outlined area. Scale bar: 10 μm. (B) Myocardial permeability assessed by Evans Blue extravasation in sham- and transverse aortic constriction–operated (TAC-operated) Nogo-A/Bf/f and EC-Nogo-A/B–deficient mice 24 hours after surgery. n ≥ 7/group. (C) Immunohistochemical staining and (D) quantification of CD45 in myocardial sections from Nogo-A/Bf/f and EC-Nogo-A/B–deficient mice at 3 days after TAC. n ≥ 5/group. Scale bar: 100 μm. (E–G) Progressive left ventricle dysfunction of Nogo-A/Bf/f and EC-Nogo-A/B–deficient mice at baseline and 3 months after TAC. n ≥ 15/group. LVDd, left ventricle end-diastolic diameter; LVDs, left ventricle end systolic diameter; FS, fractional shortening. Data are expressed as mean ± SEM. **P < 0.01, ***P < 0.001. Statistical significance was determined by (B) 1-way ANOVA followed by Tukey’s multiple comparison test, (D) unpaired t test, or (E–G) 2-way ANOVA followed by Tukey’s multiple comparison test.