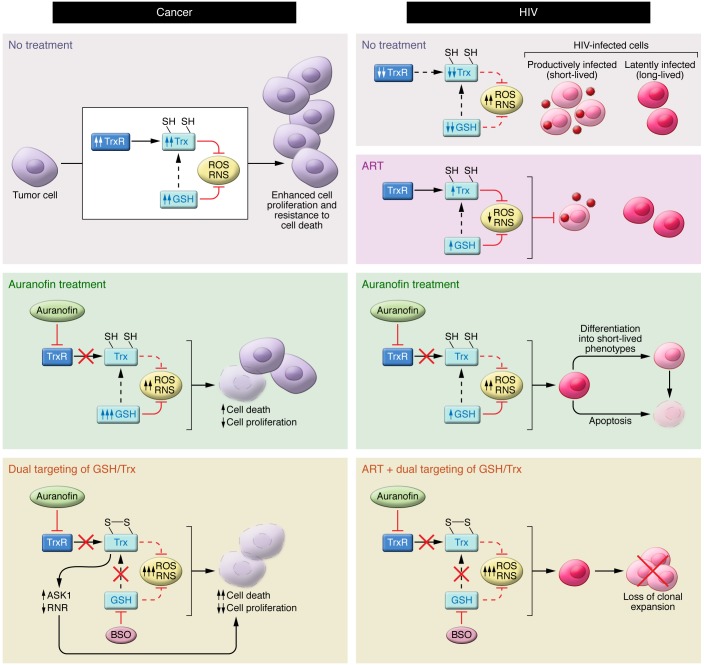
Figure 2. Model of the anticancer and anti-HIV effects of targeting the Trx and GSH systems.
Left: Many cancer cells generate large amounts of ROS and RNS but also have elevated antioxidant defenses, largely through upregulation of the Trx and GSH systems. Inhibition of TrxR by auranofin can lead to elevated ROS and RNS levels, apoptosis, and decreased cell proliferation. However, when TrxR is inhibited, the GSH redox system often becomes upregulated, eliminating ROS and RNS and acting as a backup system to reduce Trx. Exposure of cancer cells to auranofin and BSO disables both Trx and GSH systems, severely compromising antioxidant capacity and leading to marked elevation of ROS and SNOs, which suppress cell proliferation and promote cell death. Cell death is in part mediated by inhibition of ribonucleotide reductase (RNR) and through activation of the proapoptotic kinase ASK1. Right: HIV-infected cells are characterized by elevated levels of ROS, coupled with decreased intracellular levels of antioxidant defenses (Trx, TrxR, and GSH). ART blocks HIV replication and partially counteracts the effects of oxidative stress, but is unable to target the latently infected cells. Inhibition of TrxR by auranofin increases oxidative (and potentially nitrosative) stress and apoptosis. TrxR inhibition also leads to a prodifferentiation effect on the latently HIV-infected cells, driving them toward short-lived phenotypes (i.e., effector memory) and, ultimately, decreasing the viral reservoir. Auranofin and BSO impair lymphocyte activation and increase HIV-infected cell death. ROS variations are documented, whereas RNS variations are predicted. Arrows beside the names of the molecules indicate the variation over time. SH, thiol.