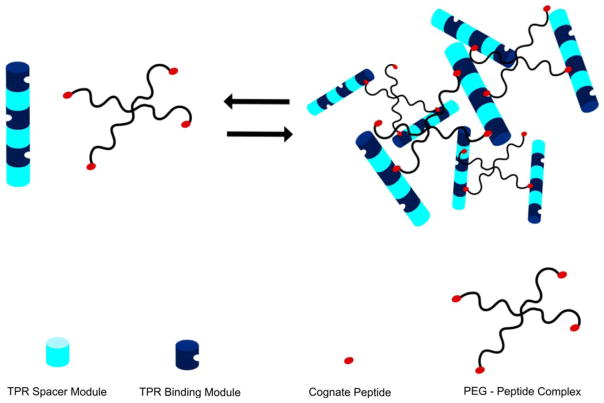
FIGURE 11.
A schematic illustration of the reversible formation of a TPR-peptide based hydrogel. Consensus TPR “binding” modules (dark blue) that bind to the cognate peptide are concatenated with TPR “spacer” modules (pale blue) that do not bind the peptide so that binding sites are arrayed on different faces of the cylinder. Peptide cross-linkers were constructed by chemical attachment of the cognate peptide to functionalized 4-armed star PEG molecules (black lines with red termini). Mixing the TPR arrays with PEG-peptide cross-linkers in a stoichiometric ratio of 1:2 results in hydrogel formation, which can be reversed by increasing ionic strength or decreasing pH.