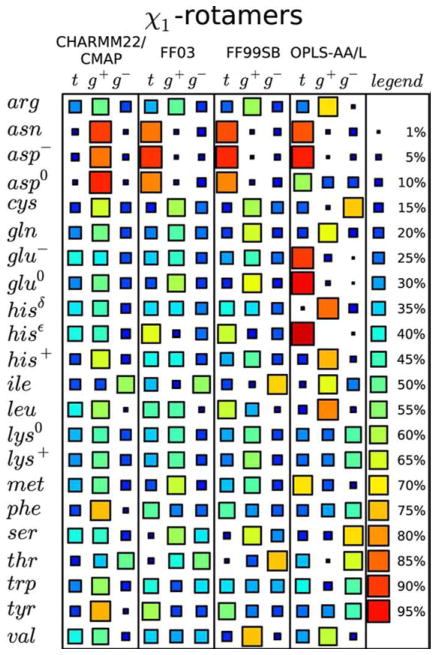
FIGURE 5.
Comparing the performance of different force-fields in predicting side-chain dihedral angle distributions. Comparison of the relative populations of χ1 dihedral angles (where t =120° <χ1 <240°, g+=240° <χ1 <360°, and g−=0° <χ1 <120°) for different amino acids resulting from simulations of short peptides in solution, using different force-fields (CHARMM22/CMAP; FF03; FF99SB; OPLS-AA/L). The relative occupancy of each side-chain dihedral is indicated by the size and color of the associated square (see legend). Each row shows the predictions for that particular amino acid type given by the specified force-field. A good “consistent” prediction would be if each force-field gave the same result. Lys is quite good. By contrast, Val is particularly bad since its results differ with each force-field. Reprinted with permission from Vymětal, J.; Vondrášek, J. Journal of Chemical Theory and Computation 2013, 9, 441–451.© 2013 American Chemical Society.