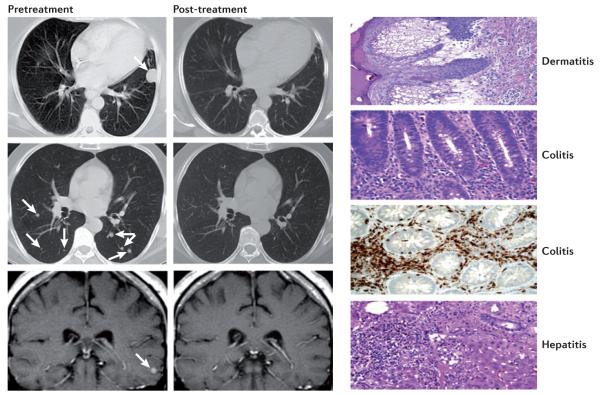
Figure 2. Clinical responses and immune-mediated toxicities on antibody blockade of the CTLA4-mediated immune checkpoint.
Depicted on the left of the figure are examples of regressions of lung (top two panels) and brain (lower panel) metastases in a patient with melanoma who was treated with the cytotoxic T-lymphocyte-associated antigen 4 (CTLA4) antibody, ipilimumab. 25–30% of patients treated with extended doses of anti-CTLA4 therapy can develop immune-related ‘on-target’ toxicities. However, the frequency of severe adverse toxicities was lower (10–15%) with the short course that was used in the Phase III trial that led to the approval of ipilimumab. This short-course regimen (4 doses at a cost of US$30,000 per dose) was recommended by the US Food and Drug Administration (FDA). As shown on the right of the figure, common tissues affected by immune-related toxicities from treatment with anti-CTLA4 therapy include the skin (dermatitis) and the colon (colitis). Tissues that do not undergo such rapid regeneration as the skin and colon, such as lung and liver and the pituitary and thyroid glands, are less frequently affected. Immune toxicities from anti-CTLA4 therapy are usually successfully mitigated by treatment with systemic steroids and tumour necrosis factor (TNF) blockers when systemic steroids are not effective. Ongoing tumour responses typically continue even after a course of steroids. Figure is reproduced, with permission, from REF. 39 © (2003) National Academy of Sciences, USA.