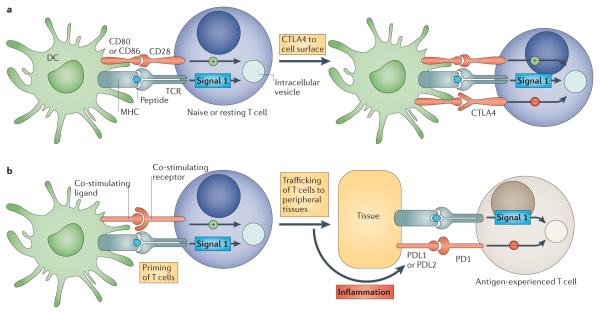
Figure 3. Immune checkpoints regulate different components in the evolution of an immune response.
a | The cytotoxic T-lymphocyte-associated antigen 4 (CTLA4)-mediated immune checkpoint is induced in T cells at the time of their initial response to antigen. The level of CTLA4 induction depends on the amplitude of the initial T cell receptor (TCR)-mediated signalling. High-affinity ligands induce higher levels of CTLA4, which dampens the amplitude of the initial response. The key to the regulation of T cell activation levels by the CD28–CTLA4 system is the timing of surface expression. Naive and memory T cells express high levels of cell surface CD28 but do not express CTLA4 on their surface. Instead, CTLA4 is sequestered in intracellular vesicles. After the TCR is triggered by antigen encounter, CTLA4 is transported to the cell surface. The stronger the stimulation through the TCR (and CD28), the greater the amount of CTLA4 that is deposited on the T cell surface. Therefore, CTLA4 functions as a signal dampener to maintain a consistent level of T cell activation in the face of widely varying concentrations and affinities of ligand for the TCR. b | By contrast, the major role of the programmed cell death protein 1 (PD1) pathway is not at the initial T cell activation stage but rather to regulate inflammatory responses in tissues by effector T cells recognizing antigen in peripheral tissues. Activated T cells upregulate PD1 and continue to express it in tissues. Inflammatory signals in the tissues induce the expression of PD1 ligands, which downregulate the activity of T cells and thus limit collateral tissue damage in response to a microorganism infection in that tissue. The best characterized signal for PD1 ligand 1 (PDL1; also known as B7-H1) induction is interferon-γ (IFNγ), which is predominantly produced by T helper 1 (TH1) cells, although many of the signals have not yet been defined completely. Excessive induction of PD1 on T cells in the setting of chronic antigen exposure can induce an exhausted or anergic state in T cells. MHC, major histocompatibility complex.