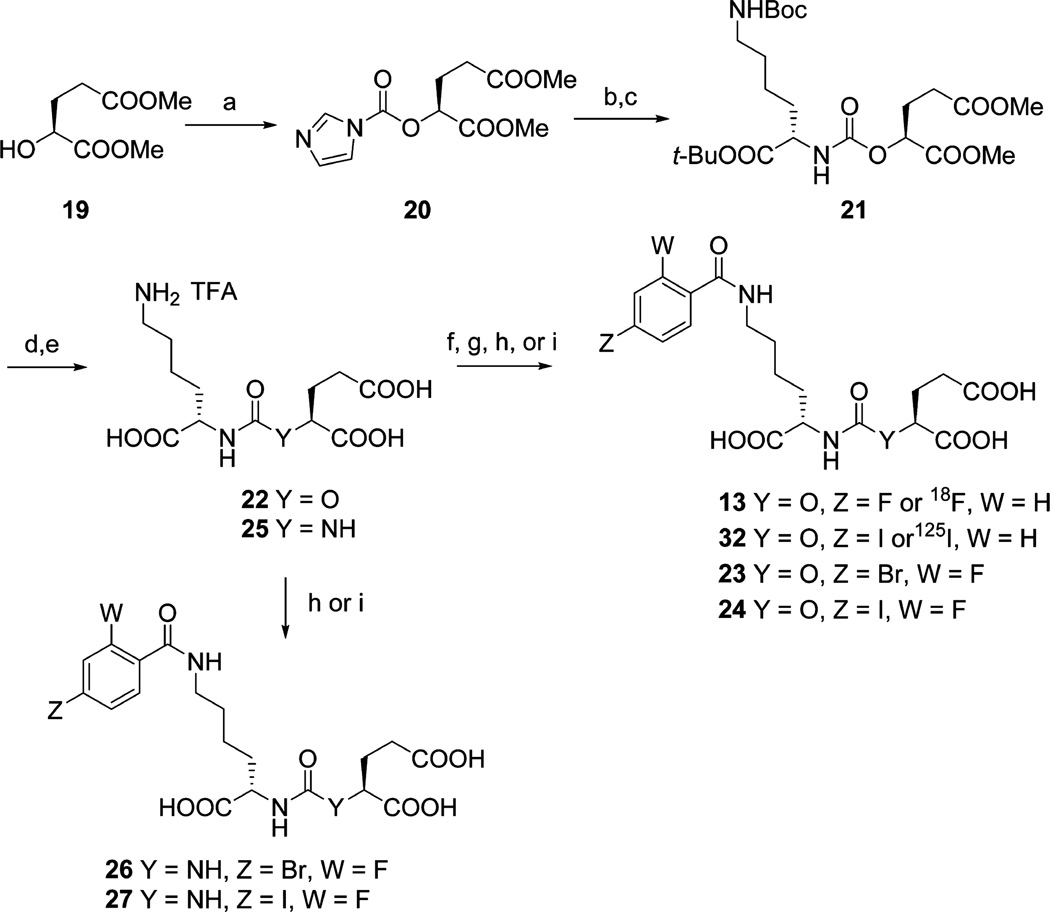
Scheme 2a.
aReagents and conditions: (a) carbonyldiimidazole, CH2Cl2, rt, 1 h; (b) Mel, MeCN, 55 °C, 3 h; (c) N-Boc-lysine-tert-butyl ester hydrochloride, Et3N, DMSO, rt, overnight; (d) TFA/CH2Cl2 (1/1), rt, 2 h; (e) LiOH, THF/H2O (1/1), rt, 4 h; (f) N-succinimidyl-4-fluorobenzoate or N-succinimidyl-4-[18F]fluorobenzoate, Et3N, DMSO, rt, 2 h; (g) N-succinimidyl-4-[125I]iodobenzoate or N-succinimidyl-4-iodobenzoate, diisopropylethylamine, DMSO, rt, 1 h; (h) N-succinimidyl 4-bromo-2-fluorobenzoate, Et3N, DMSO, rt, 2 h; (i) N-succinimidyl 4-iodo-2-fluorobenzoate, Et3N, DMSO, rt, 2 h.