Fig. 1.
. Disposition of individuals at Week 48.
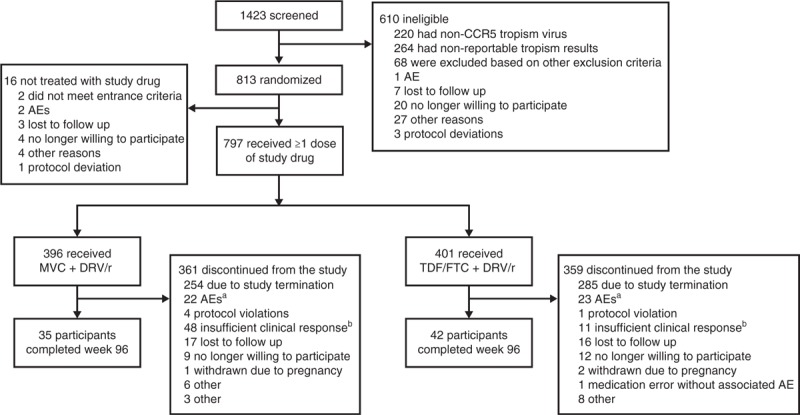
AE, adverse event; MVC, maraviroc; DRV/r, darunavir/ritonavir; TDF/FTC, tenofovir/emtricitabine. aOf the AEs leading to discontinuation, 12 in each group were considered to be related to study treatment. bInvestigator decision to discontinue for lack of efficacy; individual did not necessarily meet criteria for protocol-defined virologic failure.
