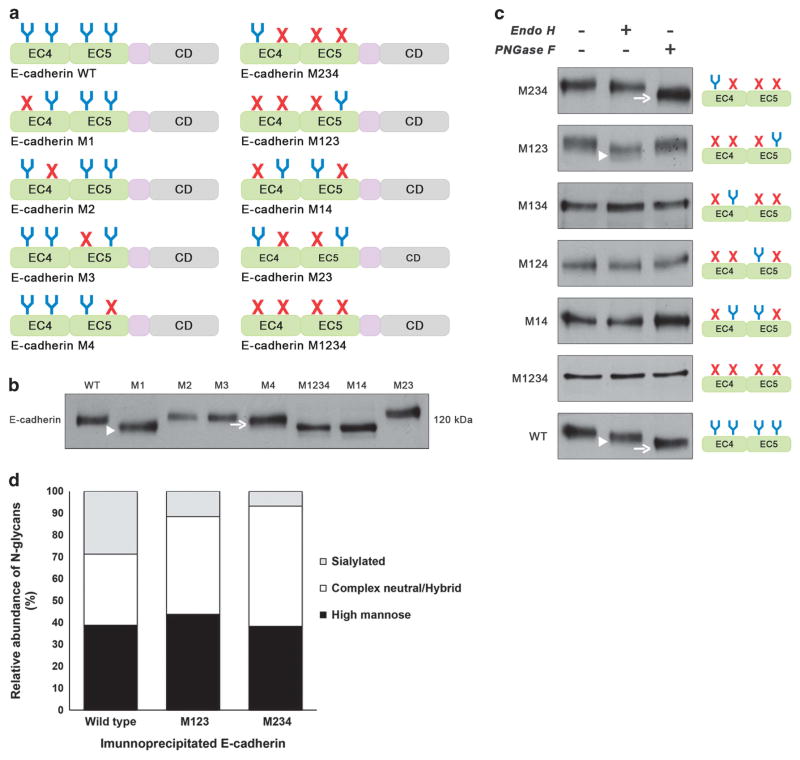
Figure 3.
N-glycosylation sites Asn-554 and Asn-633 are N-glycosylated being occupied with complex-type N-glycans and high-mannose/ hybrid- type N-glycans, respectively. (a) Schematic representation of different E-cadherin N-glycan mutants lacking selected putative N-glycosylation sites by replacing asparagine residue with glutamine in each N-glycosylation consensus sequence (NXS/T) either individually or in different combinations. EC4, subdomain EC4; EC5, subdomain EC5; CD, cytoplasmic domain; E-cadherin WT, E-cadherin wild type; E-cadherin M1, lacking Asn-554; M2, lacking Asn-566; M3, lacking Asn-618; M4, lacking Asn-633; M14, lacking Asn-554 and Asn-633; M23, lacking Asn-566 and Asn-618; M234, lacking Asn-566, Asn-618, and Asn-633; M123, lacking Asn-554, Asn-566, Asn-618; M1234, lacking Asn-554, Asn-566, Asn-618, and Asn-633. (b) Evaluation of the mobility shift of E-cadherin N-glycan mutants in AGS cells compared with WT. E-cadherin N-glycan mutant M1 and M4 showed an increased mobility compared with E-cadherin WT. No detectable changes were observed regarding cadherin M2 and M3. E-cadherin M1 (arrowhead) displayed a higher mobility shift than E-cadherin M4 (arrow). (c) Characterization of N-glycosylation profile of E-cadherin N-glycan mutants in AGS cells. Total cell lysates from selected transfectants were treated with Endo H and PNGase F and analyzed for mobility shifts by western blotting. E-cadherin WT was sensitive to Endo H (arrowhead) and PNGase F (arrow) being modified with high-mannose-, hybrid- and complex-type N-glycans. E-cadherin M234 was mostly sensitive to PNGase F (arrow) and resistant to Endo H while E-cadherin M123 was predominantly Endo H-sensitive (arrowhead). See also Supplementary Figure S3. (d) N-glycomics analysis using PGC nanoLC ESI MS/MS analyses showed E-cadherin WT to carry high-mannose, complex neutral and complex sialylated N-glycans in similar amounts. E-cadherin WT and M234 showed higher levels of sialylated and complex-type N-glycans, respectively. The data presented is referred to one biological replicate, which according to the well-established methodology, can be considered reproducible and significant. See also Supplementary Figure S2 and Supplementary Tables S1 and S2.