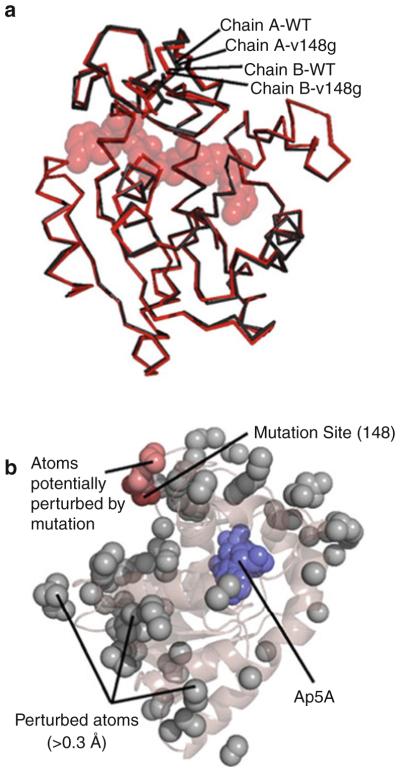
Fig. 4.
The ground state structure of AK(e) is unaffected by entropy-promoting LID mutations. (a) Structural alignment of the experimentally determined crystal structures of wild-type (PDB ID 3HPQ) and mutant V148G (PDB ID 3HPR). Shown in red are chains of each protein from positions A of their respective asymmetric units, and shown in black are chains from positions B. Substrate analog Ap5A is shown in spacefill. (b) Analysis of possible structural perturbations due to mutation. The gray spheres represent all atoms that move >0.3 Åfrom the wild-type to the mutant structure in both copies within the asymmetric unit. The dark red spheres show the mutation site V148G. The light red spheres show all perturbed atoms (gray) that can be connected to the mutation site by a continuous chain of no more than 6 Åper step of other perturbed atoms. Ap5A is shown in blue spacefill