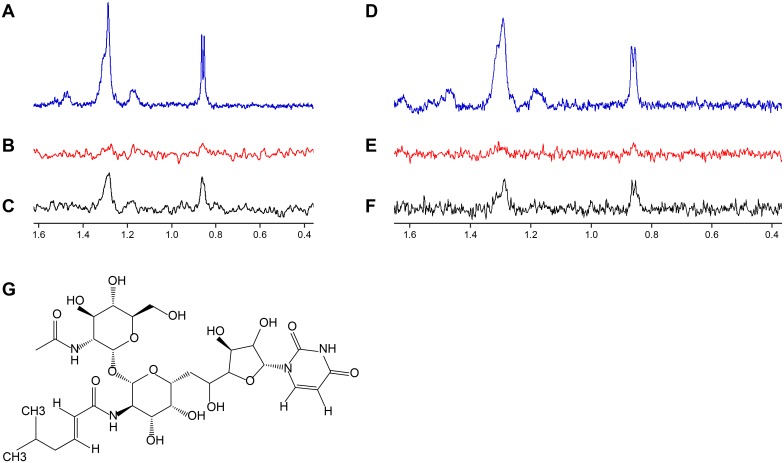
Fig 7. Biophysical studies demonstrate MnaA and Cap5P bind tunicamycin.
(A, D) 600 MHz 1H NMR spectra of 15 μM tunicamycin. (B, E) 1H NMR STD spectra of 15 μM tunicamycin without 2-epimerase. (C) 1H NMR STD spectra of 15 μM tunicamycin in presence of 5 μM MnaA. (F) 1H NMR STD spectra of 15 μM tunicamycin in presence of 5 μM Cap5P. Saturation of the protein was achieved with a Gaussian pulse cascade resulting in a total saturation time of 3s. The protein resonances were saturated at 100 Hz and the off resonance was set to -120 ppm. Tunicamycin-specific peaks in NMR STD spectra were only obtained in the presence of MnaA or Cap5P. (G) Structure of tunicamycin.