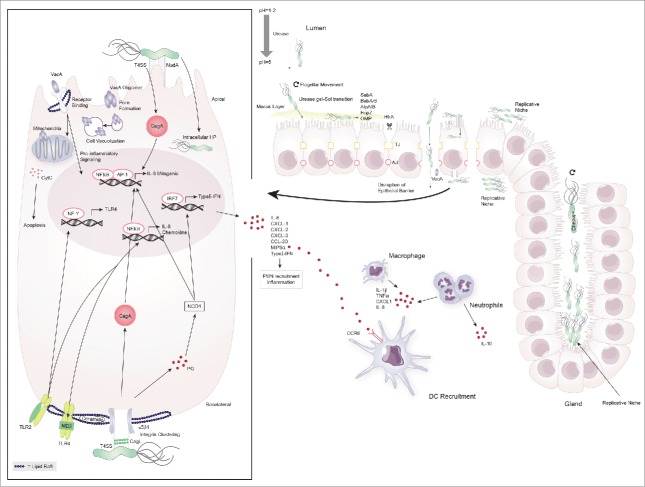
Figure 1.
Colonization of the gastric niche and initiation of immune responses by H. pylori. H. pylori possesses a number of virluence factors that aid in its ability to colonize and exploit the gastric niche for survival and replication. It elevates the gastric pH by secretion of urease and traverses the mucus layer through flagellar movement and urease-induced gel-sol transition. Once in contact with the epithelial cell layer it binds to the apical cell surface using various adhesins (SabA, BabA/B, AlpA/B, HopZ, OMP) causing cell damage and facilitating the delivery of toxins. H. pylori disrupts the cell barrier by breaking up tight- and adherent junctions (TJ, AJ) through HtrA and enters the lamina propria to replicate at the basolateral cell surface. H. pylori further exploits gastric glands and the apical cell surface as replicative niche for which the proteins Chepep and the virulence factor CagA respectively are essential. At the basolateral cell surface H. pylori forms the type 4 secretion system (T4SS) encoded by the cag pathogenicity island and causes integrin clustering at lipid rafts to inject CagA into epithelial cells. Peptidoglycan (PG) leaks through the T4SS and is recognized by the pathogen recognition receptor (PRR) NOD1. These two virulence factors induce the transcription of host-cell genes including IL-8, chemokines and type-I interferons (type-I IFN) through NFκB/AP1 and IRF7, respectively. H. pylori further causes host cell remodeling and damage through VacA. This toxin either heterodimerizes and forms pores in the cell membrane or enters by receptor binding to cause cell-vacuolization, pro-inflammatory cytokine signaling and cytochrome C (CytC)- induced apoptosis. Epithelial cells at the mucosal barrier are involved in initiating the immune response to H. pylori by antigen-induced TLR-2 and -4 signaling through NFκB. This is further amplified by the induction of TLR-4 transcription via NF-Y-mediated TLR-2 signaling. The subsequent secretion of chemokines attracks peripheral mononuclear cells (PMN) including neutrophils and dendritic cells to the site of infection.