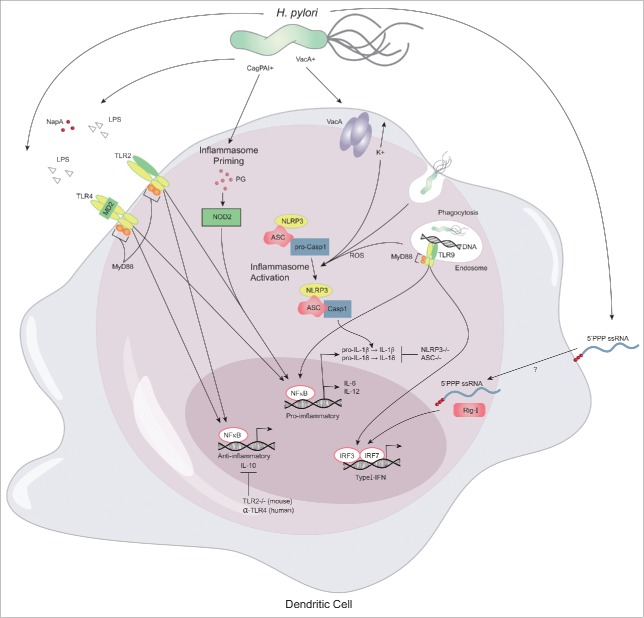
Figure 2.
Innate immune sensing of H. pylori. The innate immune response to H. pylori is initiated by epithelial cells and tissue resident macrophages. Upon chemokine secretion dendritic cells are recruited to the gastric mucosa, where they encounter, recognize and process various known and unknown H. pylori antigens. Myd88-dependent TLR2 (LPS, NapA) and TLR4 (LPS) antigen binding results in both pro- and anti-inflammatory cytokine production which reflects the complexity of the immune response initiated by H. pylori. Following phagocytosis bacterial DNA is bound by TLR9 on endosomes and induces transcription of pro-inflammatory cytokines and type-I interferons (IFN) through NFkB and IRF7/IRF8, respectively. Bacterial RNA is recognized by RIG-I and elicits Myd88-independent signaling to induce transcription of type-I IFN. Furthermore, inflammasome priming is mediated by binding of peptidoglycan (PG) to NOD2 and subsequent activation of NFκB-mediated transcription of pro-IL-1β. Subsequently, the inflammasome complex NLRP3/ASC/pro-Casp1 is activated by potassium efflux and phagocytosis-induced ROS production, which results in Casp1-mediated cleavage of pro-IL-1β to IL-1β.