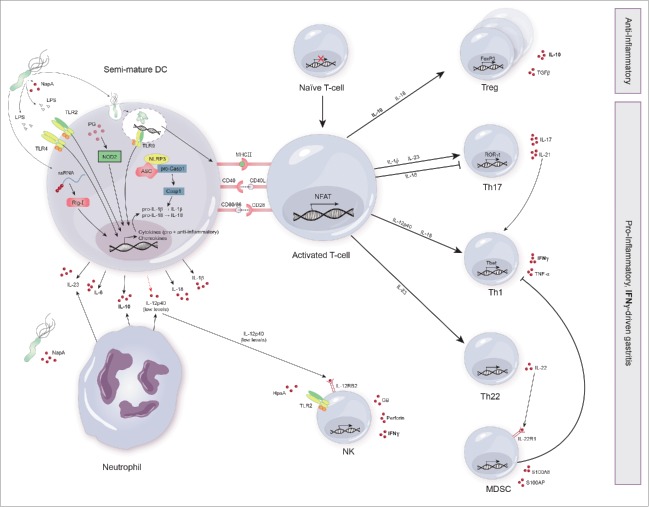
Figure 3.
Shaping adaptive immune responses to H. pylori. Dendritic cells (DC) encountering H. pylori have been shown to possess a semi-mature phenotype. The adaptive immune response to H. pylori is characterized by a mixed anti- and pro-inflammatory response. Upon pathogen receptor recognition, DC and neutrophils secrete a number of cytokines including IL-1β, IL-6, IL-10, IL-18, IL-23 and low levels of IL-12p40. These cytokines and concomitant presentation of H. pylori antigens by semi-mature DC to naive T cells via MHC-II results in the expansion of H. pylori-specific Treg. Furthermore, T cells differentiate into Th17, Th1 and Th22. The secretion of IL-22 by Th22 induces the secretion of anti-bacterial proteins by MDSC (monocytic-myeloid derived suppressor cells). IL-21 secretion by Th17 supports Th1 differentiation and thus maintains the Th17/Th1 axis during gastritis. In contrast, MDSC exert an inhibitory effect on Th1 cells. Upon binding of HpaA to TLR2 and IL-12RB2 signaling, NK cells secrete granzyme B (GB), perforin and IFN-γ. Importantly, IFN-γ secreted by NK cells, Th1 cells and possibly other cell types (CD8+ T cells) has been identified as the main driver of gastritis.